Understanding Lewis Lung Cancer and T Cell Exhaustion: Mechanisms, Implications, and Therapeutic Approaches
- The Lewis Lung Carcinoma Model: An Overview
- T Cell Exhaustion: Definition and Immunological Significance
- How Lewis Lung Cancer Induces T Cell Exhaustion
- Key Molecular Pathways in T Cell Exhaustion: A Comparative Table
- Tumor-Infiltrating Lymphocytes (TILs) in the Lewis Lung Model
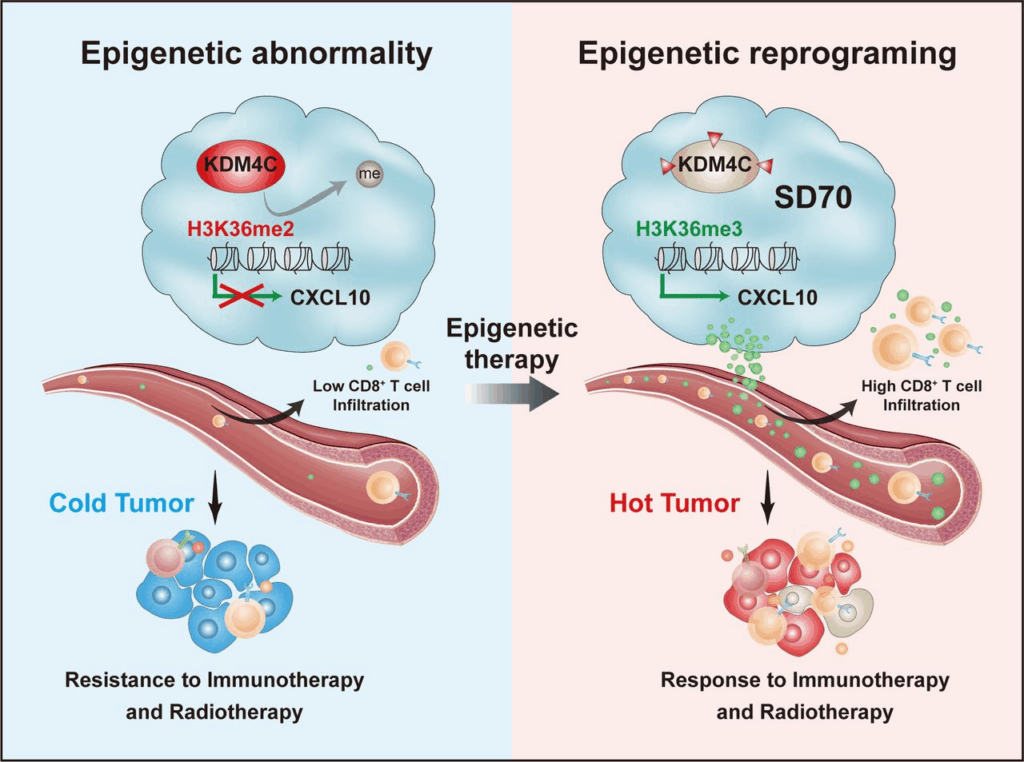
- Epigenetic Regulation of Exhausted T Cells
- Immunotherapy Resistance in Lewis Lung Carcinoma
- Impact of Hypoxia on T Cell Function in Tumors
- The Role of Regulatory T Cells in Tumor Immune Suppression
- Adoptive Cell Therapy in Lewis Lung Cancer
- Metabolic Modulation as a Strategy to Combat T Cell Exhaustion
- Vaccine-Based Approaches in the LLC Model
- Comparative Overview of T Cell Dysfunction Parameters in LLC vs. Other Models
- Microbiome Influence on T Cell Exhaustion
- Biomarker Discovery for Predicting T Cell Exhaustion
- Future Research and Translational Relevance
- FAQ
The Lewis Lung Carcinoma Model: An Overview
A Critical Tool in Cancer Immunology Research
The Lewis lung carcinoma (LLC) model was first developed in the 1950s from a spontaneous lung tumor in a C57BL mouse. It has since become one of the most extensively used murine models in oncology, particularly for studying non-small cell lung cancer (NSCLC). The model is syngeneic, meaning that tumor cells can be transplanted into immunocompetent mice without the risk of immune rejection, which makes it an indispensable tool for evaluating host-tumor immune interactions.
One of the defining features of the LLC model is its aggressive growth pattern and metastatic potential, primarily to the lungs. Its rapid progression and well-defined histological characteristics make it suitable for preclinical drug testing and immune checkpoint research. Researchers utilize this model to assess the role of immune evasion, tumor microenvironment remodeling, and mechanisms of T cell dysfunction, especially exhaustion.
Importantly, the LLC model reflects the immune suppression seen in many human cancers, allowing for investigation into why immunotherapy often fails in certain patients. Its application spans from basic molecular biology to translational drug development, helping to bridge the gap between bench research and clinical practice.
T Cell Exhaustion: Definition and Immunological Significance
Functional Decline in Chronic Disease and Cancer
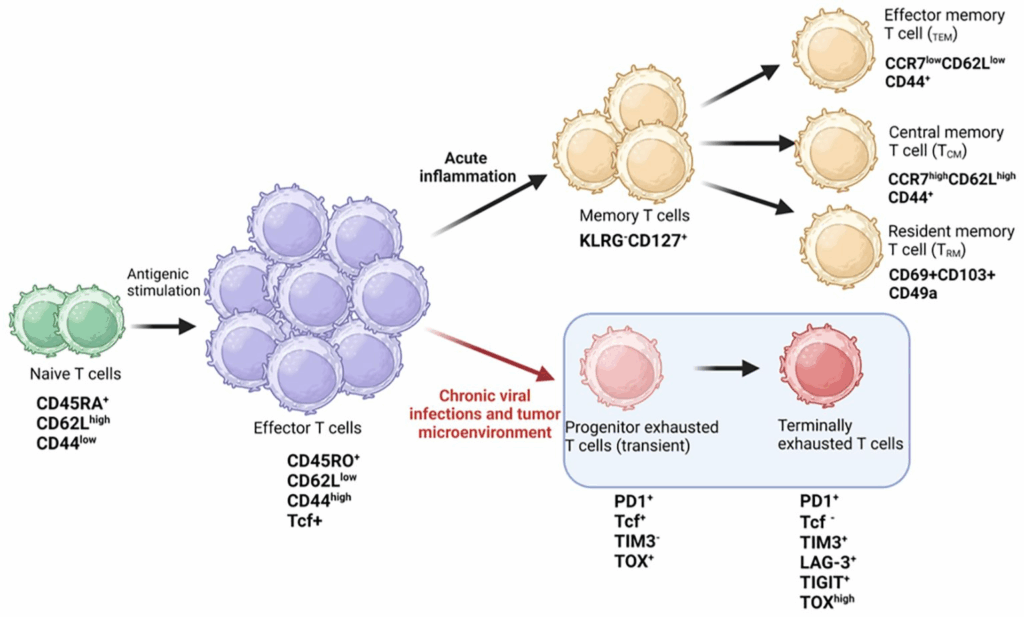
T cell exhaustion refers to a dysfunctional state that arises when T cells are exposed to persistent antigen stimulation, such as in chronic infections or cancer. Rather than being acutely activated and then resolving, exhausted T cells fail to effectively proliferate, produce cytokines, or kill tumor cells. This condition is marked by the progressive loss of effector functions and the sustained expression of inhibitory receptors such as PD-1, LAG-3, and TIM-3.
In the context of cancer, particularly in solid tumors like Lewis lung carcinoma, T cell exhaustion is driven by ongoing tumor antigen presence combined with a suppressive tumor microenvironment. Factors such as regulatory T cells, myeloid-derived suppressor cells, hypoxia, and nutrient deprivation exacerbate this state, leading to immune evasion.
Exhausted T cells are not simply “inactive.” They exhibit unique transcriptional and epigenetic profiles, distinguishing them from anergic or senescent cells. Understanding these distinctions is crucial for developing targeted therapies aimed at reinvigorating the anti-tumor immune response. As is similarly emphasized in discussions about pili multigemini cancer, where recognizing atypical biological behavior aids proper diagnosis, identifying T cell exhaustion accurately is key to effective immunotherapy.
How Lewis Lung Cancer Induces T Cell Exhaustion
Tumor Microenvironment and Immune Checkpoints
The Lewis lung carcinoma model offers a valuable window into the causes of T cell exhaustion. When tumor cells are introduced into mice, the resulting tumor microenvironment (TME) becomes a complex immunosuppressive zone. This includes the upregulation of ligands for inhibitory receptors such as PD-L1, which binds to PD-1 on T cells and blocks their activation.
In the LLC model, researchers observe that tumor-infiltrating lymphocytes (TILs) often exhibit high levels of PD-1 and other exhaustion markers. Moreover, these TILs produce reduced amounts of IFN-γ and TNF-α, key cytokines required for cytotoxic activity. The presence of suppressive immune cells, such as Tregs and MDSCs, further amplifies dysfunction by secreting TGF-β and IL-10, which inhibit T cell signaling.
Additionally, metabolic stress within the tumor environment contributes significantly. Tumor cells outcompete T cells for essential nutrients like glucose and amino acids, effectively starving them into dysfunction. As in the early stages of eyelid cancer, where subtle but progressive signs require close observation, the initial immunological changes in T cell behavior need to be studied in fine detail to intervene effectively.
Key Molecular Pathways in T Cell Exhaustion: A Comparative Table
To understand and target T cell exhaustion, researchers analyze specific molecular signaling pathways that regulate this dysfunctional state. Below is a comparison of the main pathways observed in Lewis lung carcinoma studies:
| Pathway | Key Molecules | Effect on T Cells | Therapeutic Target Potential |
| PD-1/PD-L1 Axis | PD-1, PD-L1 | Inhibits TCR signaling, reduces cytokine release | Immune checkpoint inhibitors |
| CTLA-4 Pathway | CTLA-4, CD80/CD86 | Competes with CD28, impairs costimulation | Anti-CTLA-4 antibodies |
| LAG-3/MHC II | LAG-3, MHC Class II | Suppresses proliferation and function | Under study, emerging therapies |
| TIM-3/Galectin-9 | TIM-3, Galectin-9 | Induces apoptosis, promotes exhaustion | Combination therapy potential |
| TOX/NR4A Transcription | TOX, NR4A family factors | Drives epigenetic programming of exhaustion | Gene regulation interventions |
Understanding how each of these pathways contributes to the loss of T cell function allows researchers to design more effective combination therapies. In integrative veterinary fields, such as those explored in alternative cancer treatments for dogs, complex systemic interactions must also be considered in therapy design.
Tumor-Infiltrating Lymphocytes (TILs) in the Lewis Lung Model
Indicators of Immune Dysfunction in Solid Tumors
Tumor-infiltrating lymphocytes (TILs) represent one of the most critical immunological components within solid tumors, including those modeled by Lewis lung carcinoma. These cells, which initially migrate to the tumor site in an effort to mount an immune response, often become functionally impaired in the face of chronic tumor antigen exposure and a hostile tumor microenvironment. In Lewis lung tumors, TILs typically present a phenotype consistent with exhaustion—marked by high expression of PD-1, TIM-3, and LAG-3, combined with reduced proliferation and cytokine output.
Detailed flow cytometry studies in the LLC model reveal that CD8+ T cells become hyporesponsive over time, particularly when exposed to upregulated PD-L1 on tumor and stromal cells. This renders the immune attack ineffective, despite the presence of sufficient T cells. Additionally, these TILs undergo metabolic reprogramming, switching to an energetically inefficient state, which compounds their dysfunction. Therapeutic strategies that aim to reinvigorate these exhausted TILs—such as immune checkpoint inhibitors or adoptive cell transfer—are being extensively studied within this model.
Epigenetic Regulation of Exhausted T Cells
Permanent Reprogramming and Challenges in Reversal
One of the hallmarks of T cell exhaustion, particularly in chronic tumors like Lewis lung carcinoma, is the epigenetic fixation of the exhausted state. Unlike transient anergy or activation-induced tolerance, exhaustion becomes hard-wired into the cell’s transcriptional landscape. This is mediated by chromatin remodeling, DNA methylation, and histone modification patterns that silence effector genes while promoting inhibitory receptor expression.
Key transcription factors such as TOX, NR4A, and BATF are pivotal in reshaping the epigenetic profile of exhausted T cells. In experimental LLC models, T cells that express these regulators show stably reduced expression of perforin, granzyme B, and IFN-γ, even when inhibitory receptor signals are blocked. This suggests that checkpoint blockade alone may not fully reverse exhaustion unless accompanied by strategies that also reprogram the epigenetic machinery.
Emerging research seeks to combine immune checkpoint inhibitors with epigenetic modulators, such as HDAC inhibitors or DNA methyltransferase inhibitors, to restore lost function. This approach underscores the complexity of reversing exhaustion once it has taken hold and highlights the need for multi-faceted therapies that target both surface receptors and internal programming.
Immunotherapy Resistance in Lewis Lung Carcinoma
Why Some Tumors Fail to Respond Despite Immune Activation
One of the more sobering findings in cancer immunology is that not all tumors respond to immunotherapy, even those with significant immune infiltration. In the case of Lewis lung carcinoma, this resistance is well-documented. Despite the application of checkpoint inhibitors such as anti-PD-1 and anti-CTLA-4, many LLC-bearing mice exhibit minimal tumor regression. This paradoxical finding has driven in-depth investigations into resistance mechanisms.
These mechanisms include non-immunogenic tumor antigens, limited neoantigen load, impaired antigen presentation by tumor cells, and immunosuppressive factors like IDO, TGF-β, and VEGF. Additionally, the tumor microenvironment in the LLC model remains highly fibrotic and hypoxic, which limits T cell trafficking and persistence. This mirrors some of the immune evasion dynamics observed in human lung cancer, making the LLC model a reliable proxy for studying therapeutic resistance.
Understanding these barriers is essential for developing combination therapies that can overcome resistance. Research is also exploring how vaccination, oncolytic viruses, or local cytokine delivery can boost the immunogenicity of LLC tumors and re-engage the immune system more effectively.
Impact of Hypoxia on T Cell Function in Tumors
Oxygen Deprivation as an Immune Suppressor
Hypoxia, or oxygen deprivation, is a defining characteristic of many solid tumors, including Lewis lung carcinoma. As tumors grow rapidly and outstrip their blood supply, regions of low oxygen develop, creating an environment that favors immune escape. Hypoxia-inducible factors (HIFs) orchestrate a wide range of cellular adaptations that ultimately impair T cell function and promote tumor survival.
Within the hypoxic zones of LLC tumors, CD8+ T cells face metabolic stress that reduces their ability to generate ATP and perform effector functions. Additionally, hypoxia promotes the expression of immune checkpoints such as PD-L1 and contributes to the recruitment of immunosuppressive cells like MDSCs. Studies in the LLC model show that targeting hypoxia—either by improving tumor oxygenation or using HIF inhibitors—can partially reverse immune suppression and enhance the effects of checkpoint blockade.
This interplay between oxygen tension and immune function reveals why tumor location and vascularization matter in treatment planning. Like identifying patterns in systemic conditions such as can cancer cause low phosphate levels, pinpointing metabolic suppressors is key to tailoring therapies that restore immune function.
The Role of Regulatory T Cells in Tumor Immune Suppression
Suppressor Cell Populations as Barriers to Therapy
Regulatory T cells (Tregs), a specialized subset of CD4+ T cells, play a fundamental role in maintaining immune homeostasis by preventing autoimmune responses. However, in the context of cancer—particularly within the Lewis lung carcinoma (LLC) model—Tregs are frequently co-opted by the tumor to suppress effective anti-tumor immunity. Their elevated presence within the tumor microenvironment (TME) has been consistently associated with poor prognosis and reduced therapeutic responsiveness.
In LLC-bearing mice, Tregs accumulate early during tumor development and migrate to the tumor site in response to chemokines like CCL22. Once in the TME, they suppress CD8+ cytotoxic T cells and dendritic cells through direct cell–cell contact and by secreting immunosuppressive cytokines such as IL-10 and TGF-β. These suppressive signals blunt T cell activation and contribute to exhaustion. Targeting Tregs through depletion strategies or functional inhibition has shown promise in restoring anti-tumor immunity in this model, although systemic removal of Tregs risks inducing autoimmunity.
Because the balance between immune suppression and effective antitumor response is delicate, combination therapies must be designed with precision. Treg targeting may be most effective when paired with checkpoint inhibitors or metabolic modifiers that simultaneously reduce exhaustion and enhance effector T cell activity.
Adoptive Cell Therapy in Lewis Lung Cancer
Engineering T Cells to Overcome Tumor Resistance
Adoptive cell therapy (ACT), particularly with engineered T cells such as TCR-T and CAR-T, represents one of the most promising immunotherapeutic strategies for overcoming T cell exhaustion in solid tumors. While CAR-T therapies have revolutionized hematological cancers, their efficacy in solid tumors like Lewis lung carcinoma remains limited due to immune evasion, poor T cell trafficking, and the suppressive TME.
The LLC model is frequently used to test ACT modalities due to its well-characterized resistance profile. One challenge observed in this model is that transferred T cells often become exhausted shortly after entering the tumor environment. This has led to the development of novel ACT designs incorporating exhaustion-resistant T cell subsets, use of cytokine pre-conditioning regimens, and CRISPR-mediated editing to knock out inhibitory receptor genes.
Additionally, researchers are exploring TIL-based ACT approaches, where tumor-reactive T cells are harvested, expanded ex vivo, and re-infused. These TILs can be primed to resist exhaustion using IL-15 and checkpoint blockade during culture. The goal is to create a robust population of effector cells capable of sustained function despite the hostile conditions of the TME.
Metabolic Modulation as a Strategy to Combat T Cell Exhaustion
Fueling T Cells for Enhanced Performance
The metabolic demands of activated T cells are significant, and within the nutrient-deprived environment of tumors such as Lewis lung carcinoma, metabolic competition severely compromises immune function. Tumor cells often consume vast quantities of glucose, amino acids, and fatty acids, depriving T cells of the energy substrates needed for cytotoxic activity.
Recent studies using the LLC model demonstrate that restoring metabolic balance can enhance T cell efficacy and delay exhaustion. Interventions such as arginine supplementation, inhibition of tumor glycolysis (e.g., using 2-deoxy-D-glucose), and enhancement of mitochondrial biogenesis in T cells have all shown promise. In addition, pharmacological agents like metformin and PI3K inhibitors are being tested to reprogram both tumor and immune metabolism in ways that favor immune activation.
This metabolic perspective adds a critical dimension to immunotherapy design, particularly as metabolic support can synergize with checkpoint blockade or ACT. It parallels systemic approaches in other complex diseases, such as those seen in alternative cancer treatments for dogs, where holistic cellular environments are considered in treatment. (ссылка на alternative cancer treatments for dogs)
Vaccine-Based Approaches in the LLC Model
Priming the Immune System to Recognize and Fight Tumors
Cancer vaccines aim to train the immune system to recognize tumor-specific antigens and initiate a robust T cell–mediated response. In the context of Lewis lung carcinoma, therapeutic vaccines have been developed and tested using peptide antigens, whole tumor lysates, and dendritic cell-based platforms. These vaccines aim to bypass the natural tolerogenic state of the immune system toward tumor antigens and create a pro-inflammatory response capable of controlling tumor growth.
One of the limitations in using vaccines alone is the rapid onset of T cell exhaustion once activated T cells reach the tumor site. For this reason, vaccines are now often combined with immune adjuvants or checkpoint inhibitors to maintain T cell functionality. The LLC model is particularly valuable for studying these combinations due to its reliable induction of exhaustion and immune resistance.
Promising results have come from vaccine strategies incorporating toll-like receptor agonists and GM-CSF to enhance antigen presentation. These studies suggest that vaccine priming, followed by immunomodulation, could become a mainstay of future cancer therapy protocols. However, the design of such therapies requires precise antigen selection and timing to avoid tolerance and enhance long-term immunity.
Comparative Overview of T Cell Dysfunction Parameters in LLC vs. Other Models
| Parameter | Lewis Lung Carcinoma (LLC) | B16 Melanoma Model | MC38 Colon Cancer Model |
| PD-1 Expression Level | High | Moderate | High |
| Tumor Antigenicity | Low | High | Moderate |
| Response to Anti-PD-1 Therapy | Poor | Moderate | Strong |
| Treg Infiltration Density | High | Moderate | Low |
| Hypoxia Level in Tumor Core | Severe | Moderate | Mild |
| Metabolic Competition Intensity | High (glucose-depleted TME) | Moderate | Low |
| CD8+ T Cell Proliferation | Suppressed | Active (early) | Sustained |
| Response to Vaccine + Checkpoint Blockade | Partial | Moderate | Robust |
This table highlights the distinct immunological challenges posed by Lewis lung carcinoma in comparison with other murine cancer models. The combination of poor immunogenicity, intense hypoxia, and severe T cell exhaustion makes the LLC model particularly useful for testing next-generation immunotherapeutics.
Microbiome Influence on T Cell Exhaustion
The Unexpected Role of Gut Flora in Lung Tumor Immunity
Recent research has unveiled an intricate link between the gut microbiome and systemic immune function, including the progression of T cell exhaustion in distant tumors like those found in the lungs. In Lewis lung carcinoma models, changes in gut flora composition—induced by antibiotics or probiotic supplementation—have been shown to alter tumor growth rates and influence T cell exhaustion phenotypes.
Mechanistically, certain gut bacteria can modulate systemic levels of immune cytokines, such as IL-12 and IL-6, which in turn shape T cell activation and persistence. Mice with a more diverse or “immunostimulatory” microbiome often show delayed exhaustion and enhanced response to checkpoint therapy. This insight has launched efforts to pair immunotherapy with microbiome modulation using fecal transplant, probiotics, or targeted bacteriophage approaches.
This systemic immuno-environmental crosstalk invites comparisons with non-tumoral conditions such as pili multigemini cancer, where underlying biological niches may influence superficial or localized disease progression.
Biomarker Discovery for Predicting T Cell Exhaustion
Tools to Guide Precision Therapy
As immunotherapies become more complex, the need for reliable biomarkers that can predict which patients (or models) will experience T cell exhaustion grows in importance. In the Lewis lung cancer model, several promising biomarkers have been identified, including circulating exosomal PD-L1, soluble TIM-3, and transcriptional signatures involving TOX and EOMES.
Identifying these markers early in treatment planning can allow oncologists to predict immune failure before it manifests clinically. This is particularly useful when considering high-cost interventions like CAR-T therapy or combined checkpoint blockade. In LLC studies, tracking exhaustion-related gene expression and receptor profiles on CD8+ T cells over time has allowed researchers to refine treatment schedules and avoid ineffective regimens.
These biomarker tools are undergoing validation in human trials and may one day be used routinely in clinical oncology, just as specific patterns help early recognition in epithelial pathologies such as early stage eyelid cancer pictures.
Future Research and Translational Relevance
Bridging Preclinical Insight and Human Treatment
The value of the Lewis lung carcinoma model lies not only in its resistance to therapy, but in its faithful mimicry of the immunosuppressive environment found in human non-small cell lung cancer. By dissecting the pathways that lead to T cell exhaustion—such as chronic antigen stimulation, metabolic starvation, and suppressive cytokine exposure—researchers are developing a more complete roadmap for overcoming these barriers.
Ongoing trials are testing combinations of checkpoint inhibitors with T cell metabolism boosters, microbiome therapies, epigenetic modifiers, and tumor vaccines—many of which were first evaluated in LLC mice. Moreover, next-generation sequencing is being used to map the clonal evolution of exhausted T cells over time, offering even deeper insights into how durable immune responses might be achieved.
While challenges remain, the convergence of basic science, translational modeling, and immunogenetics offers new hope for reversing T cell exhaustion and achieving long-term remission in aggressive lung cancers. In much the same way that holistic and multimodal therapies are being explored in alternative cancer treatments for dogs, integrated strategies may be key to success in immunotherapy.
FAQ
What is the Lewis lung carcinoma (LLC) model?
The Lewis lung carcinoma model is a widely used mouse model for studying lung cancer. It is known for its aggressive growth, resistance to immunotherapy, and ability to closely mimic human non-small cell lung cancer. Researchers use it extensively to investigate tumor progression, metastasis, and immune interactions within the tumor microenvironment.
How does T cell exhaustion occur in Lewis lung cancer?
T cell exhaustion in LLC occurs due to persistent antigen exposure, chronic inflammation, and an immunosuppressive tumor microenvironment. Exhausted T cells exhibit diminished cytokine production, upregulated inhibitory receptors like PD-1, and reduced cytotoxic function, making them less effective in attacking cancer cells.
Why is the LLC model resistant to checkpoint inhibitors?
LLC’s resistance to checkpoint inhibitors stems from a combination of low tumor antigenicity, high levels of regulatory T cells, and a hypoxic, metabolically hostile environment. These factors together prevent sufficient activation and persistence of effector T cells, even when PD-1/PD-L1 interactions are blocked.
What are key markers of T cell exhaustion in LLC?
Markers include elevated expression of inhibitory receptors like PD-1, LAG-3, TIM-3, and transcription factors such as TOX and EOMES. These indicators are commonly found on dysfunctional CD8+ T cells within LLC tumors and help identify exhausted phenotypes.
Can T cell exhaustion be reversed in LLC?
T cell exhaustion can be partially reversed using strategies such as checkpoint blockade, metabolic reprogramming, or adoptive T cell therapies. However, complete functional restoration remains challenging, especially in highly suppressive environments like those in the LLC model.
How does metabolic stress contribute to exhaustion?
In the LLC model, tumor cells consume key nutrients like glucose and amino acids, depriving T cells of energy needed for activation and persistence. This metabolic competition promotes mitochondrial dysfunction and accelerates the onset of exhaustion.
What role do regulatory T cells play in LLC?
Regulatory T cells suppress anti-tumor immunity by secreting immunosuppressive cytokines and inhibiting CD8+ T cell function. Their high presence in LLC tumors is a major contributor to immune evasion and resistance to immunotherapy.
How is the microbiome connected to T cell exhaustion?
The gut microbiome influences systemic immune tone and cytokine balance. In LLC-bearing mice, specific microbial compositions correlate with either increased or decreased T cell exhaustion, suggesting that modulating the microbiome could enhance therapy.
Are vaccines effective in reversing exhaustion in LLC?
Cancer vaccines can help prime anti-tumor responses, but their effect is often limited in LLC due to the rapid onset of T cell exhaustion. When paired with checkpoint inhibitors or immune adjuvants, their efficacy can improve significantly.
What is adoptive cell therapy’s role in LLC?
Adoptive cell therapy, including CAR-T and tumor-infiltrating lymphocyte (TIL) approaches, is being tested in LLC to overcome exhaustion. These strategies aim to introduce T cells that are less susceptible to exhaustion or are genetically modified to resist suppressive cues.
How does hypoxia affect T cell performance?
Hypoxic conditions in LLC tumors impair T cell metabolism, reduce proliferation, and increase the expression of exhaustion markers. Targeting hypoxia-related pathways is a developing area of therapeutic interest to enhance immune function.
Can early detection of exhaustion guide treatment?
Yes, biomarkers such as PD-1 levels or gene expression profiles can predict the onset of exhaustion and help oncologists tailor immunotherapy strategies before resistance fully develops.
What are current combination strategies for LLC?
Combinations of anti-PD-1 antibodies with metabolic enhancers, cytokine therapies, or Treg inhibitors are being explored in LLC to restore T cell function and prolong survival.
Is LLC a reliable model for human lung cancer?
While no animal model is perfect, LLC shares several characteristics with human lung cancer, including immune suppression and resistance to therapy, making it a valuable preclinical tool.
What future therapies show promise for reversing exhaustion?
Emerging therapies include next-gen checkpoint inhibitors, epigenetic drugs targeting TOX pathways, and microbiome-based immunomodulators. These are currently under investigation in LLC and other solid tumor models.









