Understanding Angiosarcoma of the Breast: Diagnosis, Treatment, and Outlook
- What Is Angiosarcoma of the Breast?
- Primary vs. Secondary Angiosarcoma: Key Differences
- Recognizing the Early Signs and Symptoms
- Risk Factors and Genetic Predispositions
- How Angiosarcoma of the Breast Is Diagnosed
- Histological Features and Grading of the Tumor
- Staging and Imaging Workup for Metastasis
- Common Sites of Metastasis and Patterns of Spread
- Treatment Planning: Role of Surgical Excision
- Comparison of Primary vs. Secondary Breast Angiosarcoma
- Chemotherapy and Targeted Therapy Options
- Radiation Therapy: Controversial but Sometimes Essential
- Living with Angiosarcoma: Patient Quality of Life
- Genetic and Molecular Research: Future Horizons
- Long-Term Follow-Up: Surveillance Strategies
- Integrative Support: Nutrition, Exercise, and Emotional Wellbeing
- FAQ
What Is Angiosarcoma of the Breast?
Angiosarcoma of the breast is a rare and aggressive type of cancer that originates in the endothelial cells lining the blood or lymphatic vessels of breast tissue. Unlike more common breast cancers that develop in the ducts or lobules, angiosarcoma affects the vascular structures, often presenting as a bruise-like area or a rapidly enlarging mass. This malignancy can be either primary—developing de novo in the breast—or secondary, arising after prior radiation therapy. Due to its vascular origin, it tends to grow and spread quickly, making early detection critical. While it represents less than 0.05% of all breast cancers, its prognosis and treatment challenges make it an important condition to understand.
Primary vs. Secondary Angiosarcoma: Key Differences
There are two primary forms of breast angiosarcoma: primary (idiopathic) and secondary (radiation-induced). Primary angiosarcoma usually affects younger women in their 20s to 40s and arises without any known cause. It typically forms deep within the breast tissue and might not involve the skin initially. In contrast, secondary angiosarcoma is more common in older women, typically emerging 5 to 10 years after receiving radiation therapy for a prior breast cancer. It often involves the skin and superficial tissues, appearing as reddish-purple lesions or patches on the irradiated area. Despite their different origins, both forms are aggressive, and differentiating between them is crucial for treatment decisions. Importantly, studies have shown that secondary angiosarcoma is increasing in incidence due to widespread use of breast-conserving therapy with radiation.
Recognizing the Early Signs and Symptoms
Angiosarcoma often presents subtly at first, making early diagnosis difficult. In primary cases, the symptoms may begin as a painless, rapidly enlarging mass in the breast, which can be mistaken for a benign lesion or even a hematoma. In secondary angiosarcoma, patients may notice discolored patches or nodules on the skin that appear bruised, red, or purplish. These lesions might become raised or ulcerate over time. Pain, although not always present initially, can develop as the tumor grows and invades nearby tissues. Because of its vascular nature, the tumor may bleed easily, and recurrent superficial bleeding can be a red flag. Anyone who has undergone prior radiation therapy and experiences unusual skin changes over the treated area should seek immediate medical evaluation.
Risk Factors and Genetic Predispositions
While most angiosarcomas of the breast are considered sporadic, several risk factors are associated with their development. Prior radiation therapy is the most well-documented risk, particularly in women treated for breast-conserving cancer therapy. Chronic lymphedema, especially in the setting of lymph node dissection, can also predispose a patient to lymphangiosarcoma—a closely related condition. Certain environmental exposures, such as vinyl chloride or arsenic, have been implicated in angiosarcoma of the liver and may have relevance in other forms. Genetically, mutations in tumor suppressor genes like TP53 or abnormalities in angiogenic signaling pathways (e.g., VEGF) may contribute to tumor initiation, although these findings remain under investigation. In some rare familial cancer syndromes, there may be a slightly elevated risk, though angiosarcoma remains exceedingly rare even in these populations.
How Angiosarcoma of the Breast Is Diagnosed
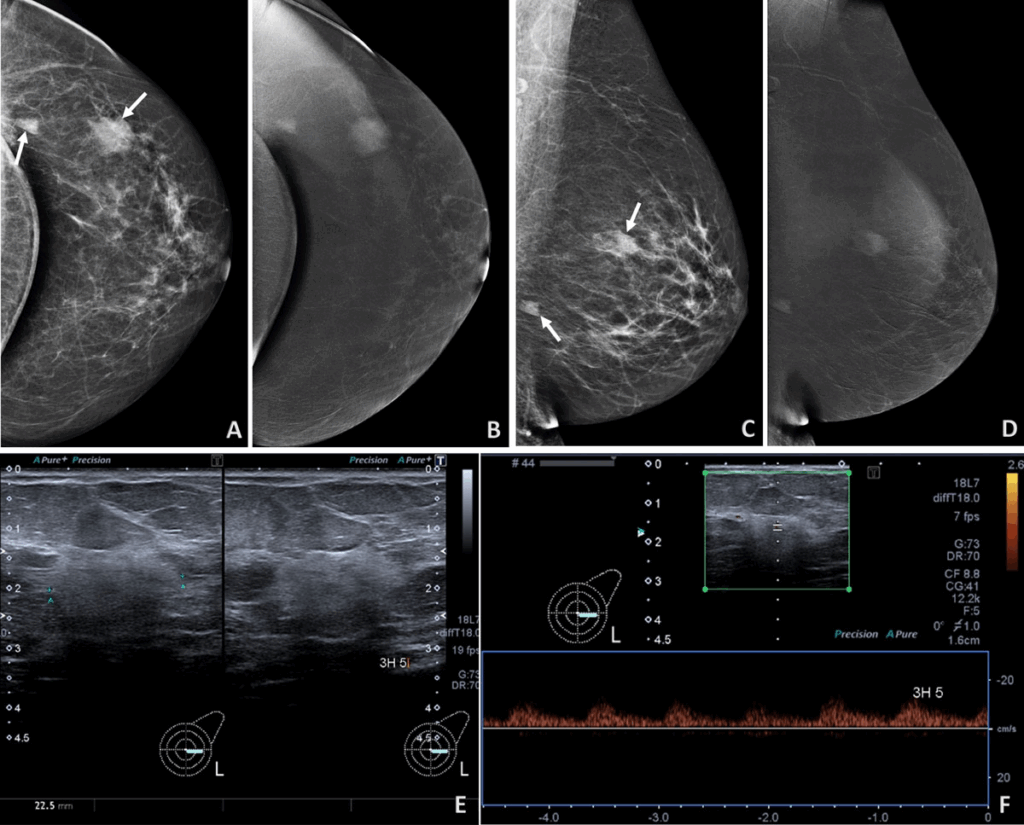
Diagnosing angiosarcoma of the breast requires a thorough clinical, imaging, and histopathological approach. Physical examination may reveal asymmetry, skin discoloration, or a palpable mass. However, since the clinical presentation often mimics benign conditions like hematomas or post-radiation skin changes, imaging is vital. Mammography may show nonspecific findings or miss deep lesions entirely, particularly in younger patients with denser breasts. Ultrasound can detect masses but cannot determine malignancy. MRI is particularly useful for identifying the extent of vascular tumors and their enhancement patterns. Ultimately, the gold standard for diagnosis is a biopsy. A core needle biopsy, rather than fine needle aspiration, is preferred due to the tumor’s complex architecture. Immunohistochemistry is then employed to confirm endothelial origin—markers like CD31, CD34, and ERG are commonly used. In difficult cases, a wide excisional biopsy might be required to establish an accurate diagnosis.
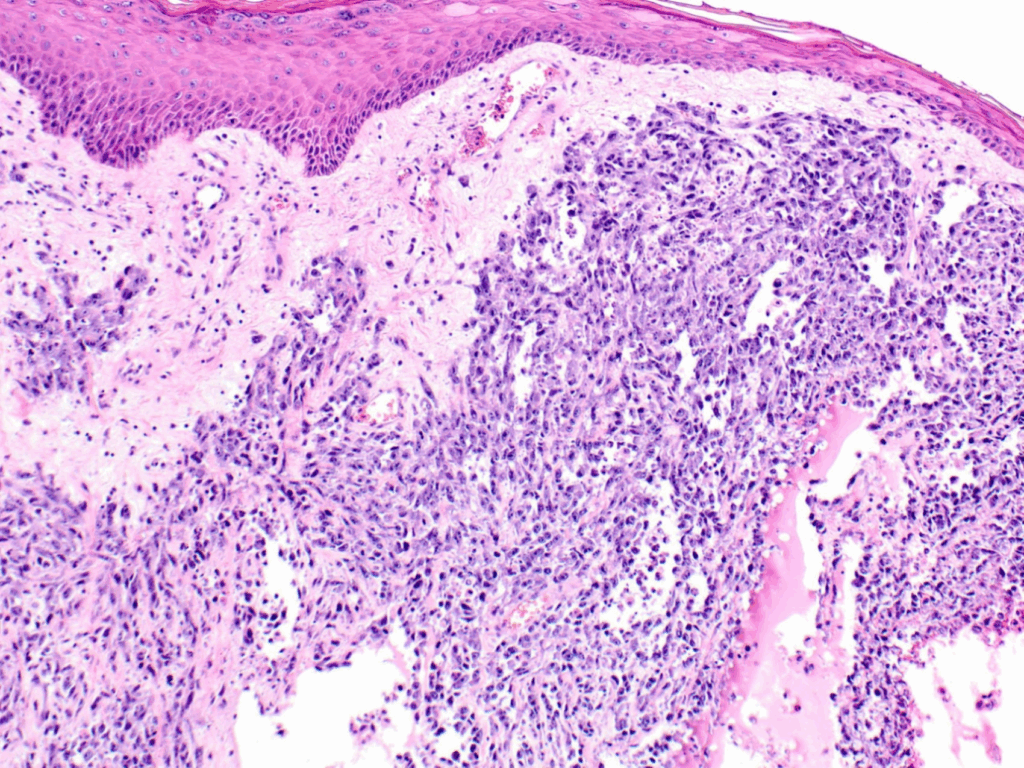
Histological Features and Grading of the Tumor
Once tissue is obtained, pathologists evaluate it under the microscope for signs of malignancy and vascular differentiation. Histologically, angiosarcoma displays a range of appearances from well-differentiated tumors with clear vascular channels to poorly differentiated, solid sheets of atypical cells. The tumor is typically graded on a scale from low- to high-grade based on mitotic activity, cellular atypia, and necrosis. Low-grade tumors may resemble benign hemangiomas, while high-grade ones appear anaplastic and are prone to rapid growth and metastasis. The grading has implications for both treatment and prognosis: higher-grade tumors often require more aggressive treatment and carry a worse outlook. Certain features like blood-filled spaces, endothelial tufting, and necrosis may help distinguish angiosarcoma from other breast cancers. Special stains and genetic tests may be used to confirm or rule out similar sarcomas or metastatic tumors.
Staging and Imaging Workup for Metastasis
Because of its high metastatic potential, staging is a crucial step once angiosarcoma is diagnosed. A complete imaging workup usually includes a contrast-enhanced CT scan of the chest, abdomen, and pelvis to evaluate for lung, liver, and peritoneal spread. PET-CT may also be performed to detect distant or occult metastases. MRI of the breast and regional nodes can help define local invasion and assist in surgical planning. Angiosarcoma can metastasize hematogenously, so bone scans or brain imaging may be considered in patients with relevant symptoms. Unlike more typical breast cancers that first spread to axillary lymph nodes, angiosarcoma spreads predominantly via the bloodstream. However, lymphatic spread is still possible and should not be ignored. Clear staging guides treatment planning and determines eligibility for clinical trials or neoadjuvant therapies.
Common Sites of Metastasis and Patterns of Spread
Angiosarcoma of the breast has a distinct pattern of metastasis compared to other types of breast cancer. Hematogenous spread is most common, and metastases are frequently found in the lungs, liver, bones, and occasionally the brain. These metastases often appear early in the disease course due to the aggressive vascular nature of the tumor. Less frequently, angiosarcoma may metastasize to the skin or soft tissue. The pattern and timing of metastasis differ between primary and secondary angiosarcoma. Primary cases may present with distant metastases at diagnosis, while secondary forms may first recur locally before systemic spread. Monitoring for metastasis is ongoing throughout treatment and follow-up, with imaging tailored to symptoms and risk.
Treatment Planning: Role of Surgical Excision
Surgery is the cornerstone of treatment for angiosarcoma of the breast, particularly in localized disease. Unlike ductal carcinomas that may be treated with lumpectomy, angiosarcoma often requires a more aggressive approach. Mastectomy is generally the preferred procedure, aiming for wide margins to minimize the risk of local recurrence. In high-grade or infiltrative tumors, achieving negative margins may be difficult, which significantly influences prognosis. Lymph node dissection is not routinely performed unless clinical suspicion exists, as nodal spread is rare. For secondary angiosarcoma, especially following radiation, surrounding skin and soft tissue excision might be necessary. Close collaboration with plastic and reconstructive surgeons is essential, especially when large resections are required. Overall, surgical planning is highly individualized and depends on tumor size, location, prior therapies, and the patient’s overall condition.
Comparison of Primary vs. Secondary Breast Angiosarcoma
| Feature | Primary Angiosarcoma | Secondary Angiosarcoma |
| Onset | Spontaneous, without prior radiation | Typically after radiation or lymphedema |
| Patient Age | Often younger (30s–50s) | Typically older (50s–70s) |
| Tumor Origin | Parenchymal breast tissue | Dermal and subdermal layers |
| Growth Pattern | Deeper, more infiltrative | More superficial with skin discoloration |
| Latency Period | None | 5–10 years after radiation |
| Prognosis | Generally worse due to aggressive behavior | Slightly better if detected early |
Chemotherapy and Targeted Therapy Options
For many patients, chemotherapy is used as either adjuvant or palliative therapy, especially in high-grade or metastatic angiosarcoma. Commonly used agents include paclitaxel, doxorubicin, and ifosfamide. These drugs can reduce tumor size and delay progression, though complete remission is rare. The choice of regimen depends on tumor grade, spread, and the patient’s tolerance for side effects. In some cases, neoadjuvant chemotherapy may be used to shrink the tumor before surgery. Research into targeted therapies is ongoing, with limited but emerging data supporting the use of anti-angiogenic agents like bevacizumab. These therapies aim to inhibit tumor blood vessel growth, a key factor in angiosarcoma pathogenesis. Clinical trials may offer access to newer agents, and their role in first- or second-line treatment is still being defined. The growing understanding of molecular markers in angiosarcoma holds promise for personalized therapy in the future.
Radiation Therapy: Controversial but Sometimes Essential
Radiation therapy plays a limited but potentially supportive role in the treatment of angiosarcoma of the breast. While it is a mainstay in many other breast cancers, its use here is controversial, especially in cases of secondary angiosarcoma that are caused by prior radiation exposure. In primary angiosarcoma, radiation may be considered postoperatively in cases with close or positive margins or when tumors are unresectable. However, evidence on survival benefit is limited. Radiation is generally avoided if the patient has already received full-dose therapy during previous breast cancer treatment. For inoperable cases, palliative radiation may help reduce tumor burden or control bleeding. Decisions around radiotherapy must be made carefully, balancing the risk of inducing new malignancy or worsening fibrosis against the potential benefits of local control.
Living with Angiosarcoma: Patient Quality of Life
Living with angiosarcoma of the breast involves navigating both the emotional and physical complexities of a rare and aggressive cancer. Patients often face significant surgical recovery and may require extensive physical therapy, especially if large portions of breast or chest wall tissue are removed. There may be changes in body image, challenges with scarring, and psychological effects related to fear of recurrence. Support groups, both in-person and online, provide emotional relief and connection with others experiencing similar struggles. Long-term follow-up care typically includes regular imaging and physical examinations, especially during the first two years, when the risk of recurrence is highest. Managing fatigue, chemotherapy side effects, and possible lymphedema is crucial to maintaining daily life. Palliative care may be introduced early, not only to manage symptoms but to support mental well-being and life planning. Addressing patient quality of life is not a secondary goal—it’s integral to the treatment journey.
Genetic and Molecular Research: Future Horizons
As with many rare cancers, understanding the genetic and molecular characteristics of angiosarcoma is central to developing future treatments. Research has identified alterations in genes involved in angiogenesis and cell cycle regulation, such as KDR, TP53, and MYC amplification, particularly in secondary angiosarcomas. These discoveries have opened the door to molecular profiling and the potential use of targeted therapies. Some preclinical studies have suggested efficacy for inhibitors of the PI3K/AKT/mTOR pathway and VEGF inhibitors. Although clinical translation has been slow due to the rarity of the disease, growing interest in personalized oncology is pushing more institutions to develop angiosarcoma-specific trials. Patients should be encouraged to consider tumor sequencing or enrollment in molecular matching studies, which may offer access to experimental therapies tailored to their tumor’s specific genetic profile.
Long-Term Follow-Up: Surveillance Strategies
Post-treatment surveillance is essential for early detection of recurrence or metastasis. In most protocols, patients undergo clinical examinations and imaging (such as MRI or PET scans) every 3 to 6 months for the first two years, then every 6–12 months thereafter. Blood tests may also be used to monitor overall health, though there are no angiosarcoma-specific biomarkers. Because recurrences often appear near the original tumor site or in the lungs, special attention is paid to chest imaging. In some cases, distant metastases to the liver or bones are also possible. Patients are encouraged to report any new skin changes, lumps, or unexplained symptoms immediately. Surveillance strategies are individualized based on original tumor grade, surgical margins, and whether radiation or chemotherapy was part of the treatment plan. Coordination between oncology, radiology, and surgical teams ensures a cohesive follow-up approach.
Integrative Support: Nutrition, Exercise, and Emotional Wellbeing
Supportive care extends beyond medications and procedures. For patients with breast angiosarcoma, integrative care can significantly improve resilience, treatment tolerance, and recovery. Nutritional guidance helps combat the side effects of chemotherapy and maintain strength, especially for those dealing with weight loss or altered taste. A diet rich in anti-inflammatory foods and adequate protein supports tissue healing and immune response. Physical activity, tailored to each patient’s capability, helps reduce fatigue and maintain mobility, especially after surgery. Emotional health is equally important. Access to psycho-oncology services, mindfulness techniques, and support networks empowers patients to cope more effectively. Some cancer centers now integrate holistic approaches, including acupuncture and art therapy, as part of their standard care. While these do not replace traditional treatment, they offer meaningful tools to support the whole person—not just the disease.
FAQ
What is angiosarcoma of the breast?
Angiosarcoma of the breast is a rare and aggressive form of cancer that originates in the blood vessels of breast tissue. Unlike more common breast cancers that begin in the ducts or lobules, angiosarcomas arise from the endothelial cells lining the blood vessels and grow rapidly, often forming a bluish or purplish mass. It can occur spontaneously (primary) or as a result of prior radiation therapy (secondary).
How is breast angiosarcoma different from typical breast cancer?
Breast angiosarcoma differs in origin, behavior, and treatment. It originates in blood vessel linings rather than breast ducts or lobules, tends to grow more quickly, and may not respond to hormone therapy commonly used in typical breast cancers. It also requires a different surgical and monitoring approach due to its aggressive nature.
What causes angiosarcoma of the breast?
The exact cause is often unknown, especially in primary angiosarcoma. However, in secondary cases, prior radiation therapy to the breast or chest wall significantly increases the risk. Chronic lymphedema is also a recognized contributing factor. Genetic mutations may also play a role in some patients, though this is still under study.
Is radiation-induced angiosarcoma more common than primary angiosarcoma?
Yes, in the context of breast tissue, secondary or radiation-induced angiosarcoma has become more commonly reported, especially in patients who had breast-conserving surgery with radiation therapy for prior breast cancer. It usually appears several years after the initial treatment.
What are the early signs and symptoms?
Early signs include discoloration of the breast skin, such as purplish, red, or blue patches that may resemble a bruise. The area can become firm or swollen and may be mistaken for inflammation. As the disease progresses, a noticeable mass or ulceration may develop, which requires immediate evaluation.
How is breast angiosarcoma diagnosed?
Diagnosis typically involves a combination of imaging (such as MRI, ultrasound, or PET/CT) and a biopsy of the suspicious area. Pathological analysis confirms the diagnosis, often requiring special staining to identify the vascular origin of the tumor. Genetic tests may also be used for detailed tumor profiling.
Can angiosarcoma be mistaken for other conditions?
Yes, in its early stages, angiosarcoma may resemble a bruise or inflammatory condition such as mastitis or cellulitis. It is often misdiagnosed until a biopsy reveals its true nature. This makes awareness and a high index of suspicion crucial in previously irradiated patients.
What treatment options are available?
Surgical removal with wide margins is the cornerstone of treatment. Depending on the tumor size and location, a mastectomy may be necessary. Radiation and chemotherapy are used selectively, and emerging targeted therapies may be considered in cases with specific genetic markers. Clinical trial enrollment is also an option.
Is chemotherapy effective against breast angiosarcoma?
Chemotherapy may be used as adjuvant, neoadjuvant, or palliative therapy. Its effectiveness varies based on tumor grade and individual patient response. Agents like doxorubicin, paclitaxel, and ifosfamide are commonly used. While not always curative, chemotherapy can reduce tumor burden or delay progression.
What is the prognosis for breast angiosarcoma patients?
Prognosis depends on multiple factors including tumor grade, size, margins, and whether it has spread. Early-stage disease with complete surgical removal has a better outcome, but the overall five-year survival rate is still lower compared to other types of breast cancer. Close follow-up is essential.
Is there a high chance of recurrence?
Yes, angiosarcoma has a high rate of local recurrence and potential for distant metastasis, particularly to the lungs, liver, and bones. This is why aggressive surgical resection and careful post-treatment monitoring are critical parts of management.
Can genetic testing help in angiosarcoma treatment?
Genetic testing can provide valuable insights, especially in recurrent or metastatic cases. It may uncover actionable mutations that guide the use of targeted therapies. Tumor profiling also contributes to ongoing research and can match patients with appropriate clinical trials.
How does follow-up care work after treatment?c
Follow-up typically involves physical exams and imaging at regular intervals—usually every 3–6 months for the first two years and then annually. Imaging may include MRI, CT, or PET scans, depending on the original tumor location and stage. Patients are encouraged to report any skin changes promptly.
Are there support groups or resources for angiosarcoma patients?
Yes, there are online forums and cancer-specific communities such as the Sarcoma Alliance, Angiosarcoma Awareness Inc., and other social media groups where patients and families can share experiences, find emotional support, and stay informed about the latest treatment options.
Can alternative therapies be part of angiosarcoma care?
While alternative therapies should never replace standard medical treatment, integrative approaches like acupuncture, nutritional support, and stress-reduction techniques can improve quality of life. These should always be discussed with the oncology team to ensure safety and coordination with ongoing treatment.









