The Eukaryotic Cell Cycle and Cancer: An In-Depth Analysis
- Introduction to the Eukaryotic Cell Cycle
- G1 Phase: Cell Growth and Preparation for DNA Replication
- S Phase: DNA Synthesis and Replication Fidelity
- G2 Phase: Pre-Mitosis Preparation and DNA Integrity Checkpoint
- M Phase: Mitosis and Cell Division (with Table)
- Cell Cycle Checkpoints and Their Role in Cancer Prevention
- Oncogenes and Tumor Suppressor Genes in Cell Cycle Regulation
- How Cancer Disrupts Normal Cell Cycle Control
- Cancer Therapies Targeting the Cell Cycle
- G1, S, G2, and M Phases: Functional Overview
- Cyclins and Cyclin-Dependent Kinases (CDKs) in Cancer Biology
- Epigenetic Regulation of Cell Cycle Genes in Cancer
- Key Molecules and Their Role in Cell Cycle Regulation and Cancer
- DNA Damage Response and Its Role in Cell Cycle Arrest
- Apoptosis and Cell Cycle Regulation in Cancer
- Therapeutic Targeting of Cell Cycle Pathways
- Future Directions: Precision Medicine and Cell Cycle Analysis
- FAQ — Common Questions About the Eukaryotic Cell Cycle and Cancer
Introduction to the Eukaryotic Cell Cycle
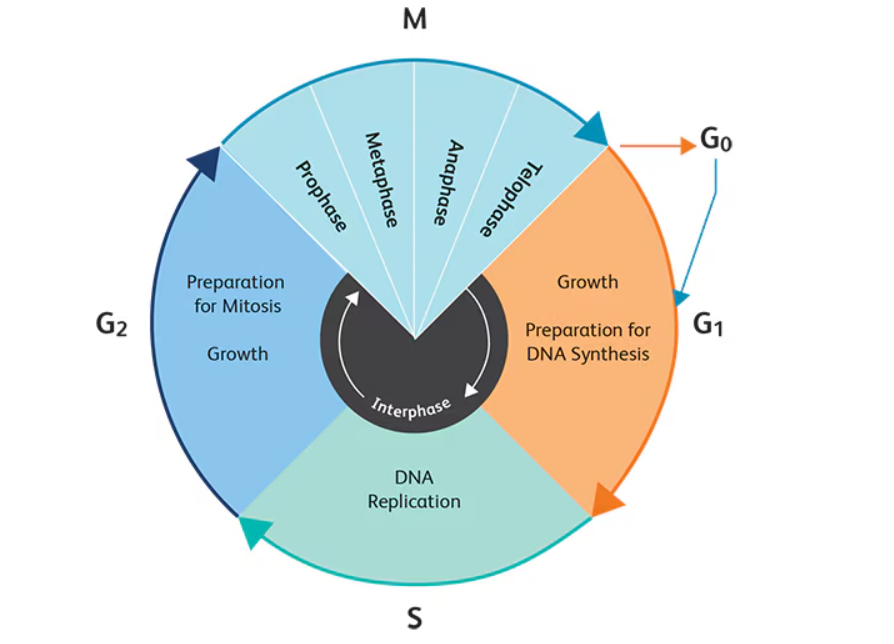
The eukaryotic cell cycle is a meticulously regulated series of events that lead to cell division and replication. It comprises distinct phases: G₁ (first gap), S (synthesis), G₂ (second gap), and M (mitosis), each with specific roles in preparing the cell for division. Interphase (G₁, S, G₂) is primarily concerned with cell growth and DNA replication, while the M phase encompasses the actual division of the cell into two daughter cells.
Regulation of the cell cycle is critical to ensure genomic integrity and proper cell function. Disruptions in this cycle can lead to uncontrolled cell proliferation, a hallmark of cancer. Understanding the mechanisms governing each phase provides insights into how normal cellular processes can go awry, leading to malignancies.
G1 Phase: Cell Growth and Preparation for DNA Replication
The G1 phase is the first stage of the interphase in the eukaryotic cell cycle and serves as a critical window during which the cell grows and prepares to duplicate its DNA. This phase occurs immediately after mitosis and before the onset of DNA synthesis in the S phase. The cell undergoes a series of biosynthetic activities, including the production of RNA, enzymes, and other proteins essential for DNA replication and cell growth.
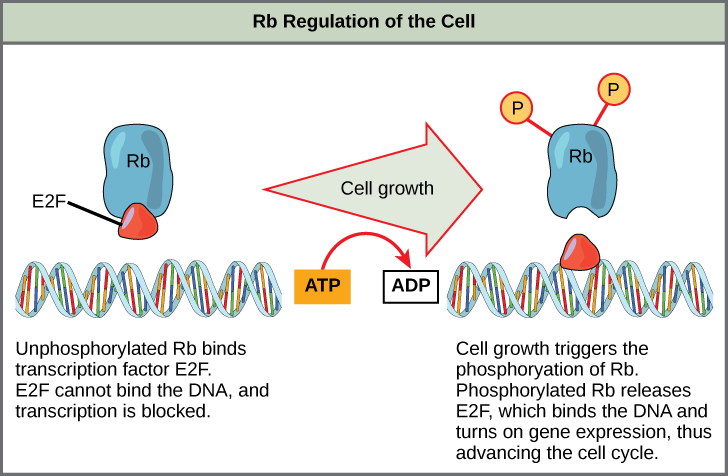
A key feature of the G1 phase is its role in cell cycle regulation. During this phase, external signals such as nutrients, growth factors, and hormones influence whether the cell will proceed to the next phase or enter a resting state (G0 phase). If favorable conditions are met, cyclin D levels rise and bind to CDK4/6, initiating the phosphorylation of the retinoblastoma protein (Rb). This phosphorylation releases E2F transcription factors, which activate the genes needed for S phase entry.
If DNA damage is detected, the G1 checkpoint is triggered. The tumor suppressor protein p53 is activated and induces the expression of p21, which inhibits cyclin-CDK complexes. This arrest allows time for repair before DNA replication begins. If the damage is beyond repair, p53 can trigger apoptosis. This mechanism highlights why p53 is often referred to as the “guardian of the genome.” When p53 is mutated, as in many cancers, cells lose this safeguard, leading to unchecked proliferation.
S Phase: DNA Synthesis and Replication Fidelity
During the S phase, the cell’s primary objective is to accurately replicate its DNA, ensuring that each daughter cell receives an exact copy of the genome. The replication process is initiated at multiple origins of replication along the DNA strands, facilitating a rapid and efficient synthesis process.
DNA polymerases, supported by helicases and primases, unwind the DNA and synthesize complementary strands. Replication is tightly regulated to prevent errors. Proofreading mechanisms and mismatch repair systems are actively engaged to ensure high fidelity. Any failure in these systems can introduce mutations, which, if they affect proto-oncogenes or tumor suppressor genes, may initiate oncogenesis.
A crucial aspect of the S phase is the duplication of centrosomes, which are vital for the formation of the mitotic spindle in later stages. Disruption in this process can lead to abnormal chromosome segregation and aneuploidy, both common features in cancerous cells. Cells with defective DNA replication may also activate the intra-S phase checkpoint, halting progression to allow for repair.
G2 Phase: Pre-Mitosis Preparation and DNA Integrity Checkpoint
The G2 phase acts as a final checkpoint before the cell commits to mitosis. During this phase, the cell continues to grow and produce proteins required for chromosome segregation and cytokinesis. More importantly, it verifies that DNA replication in the S phase has been completed successfully and without errors.
Key regulatory proteins in this phase include cyclin B and CDK1. Together, they form the maturation-promoting factor (MPF), which is inactive when DNA is damaged. The ATM and ATR kinases monitor DNA for damage or incomplete replication. If issues are detected, they activate Chk1 and Chk2, which inhibit CDC25 phosphatases, preventing MPF activation and delaying mitosis.
Failure to maintain G2 checkpoint integrity can lead to mitotic catastrophe, a phenomenon in which cells divide with damaged DNA or unreplicated chromosomes. This is a hallmark of many aggressive cancers, underscoring the checkpoint’s role in genome preservation.
M Phase: Mitosis and Cell Division (with Table)
The M phase encompasses mitosis (nuclear division) and cytokinesis (cytoplasmic division). It is the final step in the cell cycle, resulting in the production of two genetically identical daughter cells. The phase is divided into distinct stages, which are summarized below:
| Stage | Description |
| Prophase | Chromosomes condense, spindle apparatus begins to form, nuclear envelope breaks. |
| Metaphase | Chromosomes align at the metaphase plate; spindle fibers attach to kinetochores. |
| Anaphase | Sister chromatids are pulled apart to opposite poles of the cell. |
| Telophase | Chromosomes de-condense, nuclear envelope re-forms around each set of chromosomes. |
| Cytokinesis | The cytoplasm divides, forming two daughter cells. |
Successful completion of the M phase ensures that each new cell has the correct number of chromosomes. Disruption in this phase, particularly errors in spindle checkpoint signaling, can lead to aneuploidy—a condition that drives tumor progression and drug resistance in several cancers.
Cell Cycle Checkpoints and Their Role in Cancer Prevention
Checkpoints are critical surveillance mechanisms that ensure the fidelity of cell division. They act like quality control stations that either allow progression through the cell cycle or halt it when errors are detected. The three main checkpoints occur at the G1/S boundary, within the S phase, and at the G2/M transition. Each has distinct molecular components but ultimately serves the same purpose—protecting the cell from accumulating mutations that can lead to cancer.
The G1/S checkpoint assesses DNA integrity before replication. The G2/M checkpoint ensures that all DNA is replicated and free of damage before mitosis. The spindle assembly checkpoint (SAC) during mitosis verifies that all chromosomes are properly attached to the spindle before anaphase proceeds.
When these checkpoints fail—often due to mutations in regulatory genes such as TP53, ATM, or CHK2—cells continue to divide even with severe genomic damage. This unchecked division is one of the foundational processes of tumorigenesis. Interestingly, checkpoint inhibitors are being explored as cancer treatments by exploiting the differences between normal and cancerous cell responses to checkpoint disruption.
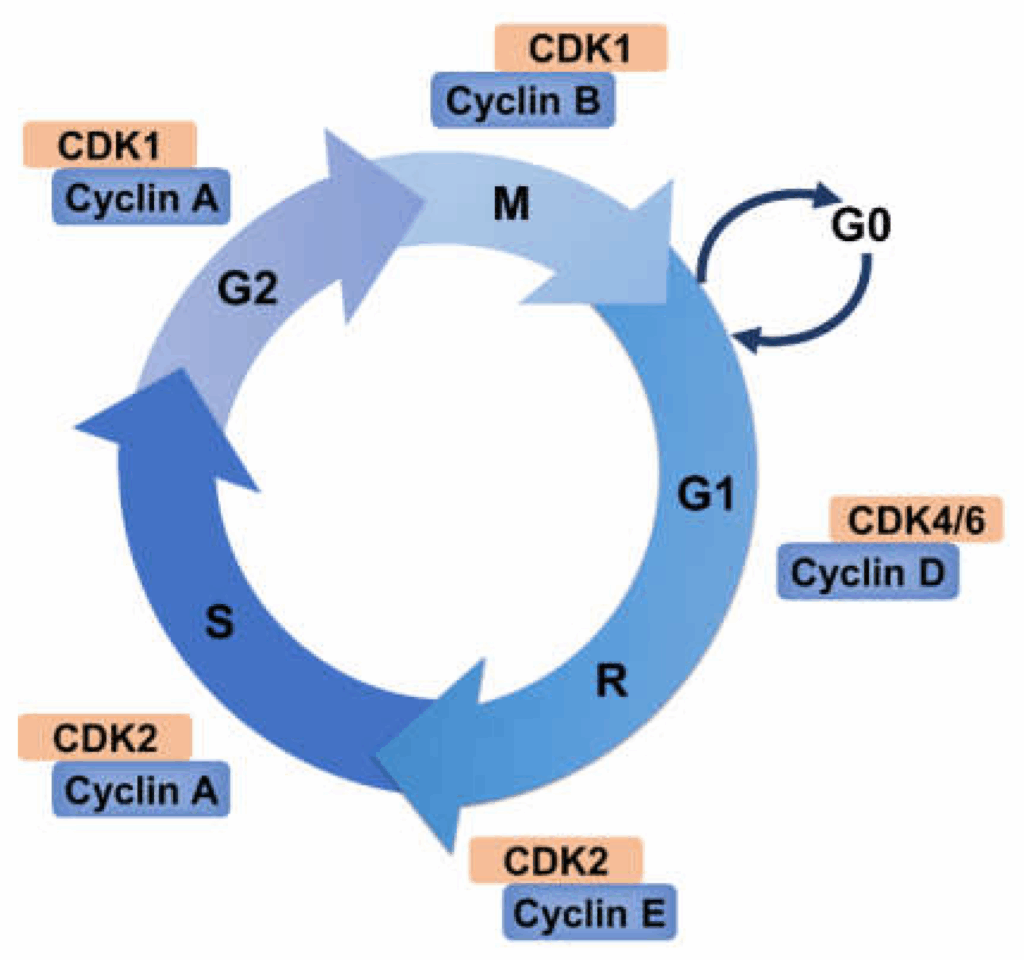
Oncogenes and Tumor Suppressor Genes in Cell Cycle Regulation
Two main categories of genes influence the cell cycle’s involvement in cancer: oncogenes and tumor suppressor genes. Oncogenes are mutated or overexpressed versions of proto-oncogenes, which normally promote cell proliferation. Examples include RAS, MYC, and HER2. When these genes become hyperactive, they drive unregulated cell division, even in the presence of DNA damage.
Tumor suppressor genes, in contrast, inhibit cell cycle progression and promote repair or apoptosis. The best-known example is TP53, which encodes the p53 protein. Another critical one is RB1, responsible for encoding the retinoblastoma protein that controls the G1/S transition. In many cancers, these genes are either deleted, silenced epigenetically, or mutated, removing essential barriers to malignant transformation.
Understanding the interplay between these gene types allows researchers to develop targeted therapies, such as tyrosine kinase inhibitors or monoclonal antibodies, that selectively disrupt cancer-promoting pathways.
How Cancer Disrupts Normal Cell Cycle Control
In healthy cells, the cycle is regulated by precise molecular interactions that ensure orderly progression. Cancer cells, however, bypass these controls through a combination of genetic and epigenetic alterations. These changes may activate oncogenes, deactivate tumor suppressor genes, or impair DNA repair mechanisms.
As a result, cancer cells exhibit several key hallmarks: sustained proliferative signaling, resistance to growth suppression, evasion of apoptosis, and replicative immortality. Many of these are direct consequences of a broken cell cycle control system. For example, the loss of functional p53 means the G1/S checkpoint is no longer enforced, allowing damaged DNA to be passed on through generations of cells.
The downstream effects include genomic instability, chromosomal abnormalities, and resistance to therapy. These disruptions not only contribute to tumor initiation but also promote progression and metastasis.
Cancer Therapies Targeting the Cell Cycle
Modern cancer treatment strategies increasingly focus on the molecular players in the cell cycle. One approach involves CDK inhibitors, such as palbociclib, which block the activity of cyclin-dependent kinases involved in G1/S transition. These are particularly effective in hormone receptor-positive breast cancers.
Other therapies aim at checkpoint kinases, especially Chk1 and Chk2, which cancer cells rely on more than healthy cells due to their underlying genomic instability. By inhibiting these kinases, therapies can push cancer cells into a crisis during division, leading to cell death.
Radiation and many chemotherapies work by causing DNA damage. Because cancer cells often have defective checkpoints, they cannot repair the damage efficiently, making them more susceptible to these treatments. However, resistance can develop, particularly when mutations restore partial checkpoint function or enhance DNA repair.
This therapeutic vulnerability emphasizes why the cell cycle remains one of the most attractive targets in oncology drug development.
G1, S, G2, and M Phases: Functional Overview
The eukaryotic cell cycle is divided into four core phases: G1 (gap 1), S (synthesis), G2 (gap 2), and M (mitosis). Each phase is tightly regulated and serves a distinct purpose.
- G1 Phase: The cell grows and prepares for DNA replication. During this time, it assesses whether the environment is favorable and whether the internal machinery is intact. If conditions are unsuitable, the cell may enter a quiescent state (G0).
- S Phase: DNA replication occurs, producing identical copies of the genome. High-fidelity polymerases and proofreading enzymes are essential here. Any replication stress or damage can initiate checkpoints.
- G2 Phase: The cell double-checks DNA replication for errors and prepares for division. Protein synthesis ramps up for mitotic structures like the spindle apparatus.
- M Phase: Mitosis and cytokinesis divide the cell into two daughter cells. Chromosomes align, segregate, and the nuclear envelope reforms.
In cancer, mutations in key regulators like cyclins, CDKs, or checkpoint proteins can lead to shortened or bypassed phases, enabling rapid but flawed division. This rapid cycle progression is especially noted in aggressive cancers such as cervical cancer and ovarian cancer.
Cyclins and Cyclin-Dependent Kinases (CDKs) in Cancer Biology
Cyclins and CDKs are central to the timing and control of the cell cycle. Different cyclins (A, B, D, E) are expressed at specific points in the cycle and activate corresponding CDKs. The cyclin-CDK complexes phosphorylate target proteins to push the cell forward.
For instance, Cyclin D binds to CDK4/6 to drive the G1/S transition, while Cyclin B with CDK1 initiates mitosis. These complexes are tightly regulated by inhibitors such as p21, p27, and p16.
In cancer, overexpression of cyclins (e.g., Cyclin D1) or loss of inhibitors (e.g., deletion of CDKN2A encoding p16) results in uncontrolled proliferation. Many cancers show aberrant cyclin/CDK activity, making them useful biomarkers and therapeutic targets. CDK4/6 inhibitors are already in use for breast cancer and are under investigation for others.
Understanding these dynamics helps researchers design drugs that can arrest cancer cells in a vulnerable phase, especially when combined with DNA-damaging agents.
Epigenetic Regulation of Cell Cycle Genes in Cancer
Epigenetic mechanisms like DNA methylation, histone modification, and non-coding RNA activity profoundly impact cell cycle control. Unlike mutations, these changes do not alter DNA sequences but can silence or activate genes.
For example, hypermethylation of the RB1 promoter can silence this tumor suppressor gene without any genetic mutation. Similarly, histone deacetylation may repress the transcription of CDK inhibitors like p21.
MicroRNAs (miRNAs) also modulate the cell cycle. Some miRNAs suppress the expression of CDKs or cyclins, while others target inhibitors. When miRNA regulation is disrupted in cancer, the balance shifts toward proliferation.
Epigenetic drugs like DNMT inhibitors and HDAC inhibitors are being developed to reverse these modifications, reactivating silenced tumor suppressors and restoring normal cell cycle arrest in cancerous cells.
Key Molecules and Their Role in Cell Cycle Regulation and Cancer
| Molecule | Normal Function | Cancer-Associated Alteration | Clinical Relevance |
| TP53 (p53) | Activates DNA repair, induces apoptosis | Mutation in >50% of cancers | Prognostic marker, target in drug trials |
| RB1 | Controls G1/S checkpoint | Deletion/methylation in retinoblastoma | Diagnostic for hereditary cancer syndromes |
| Cyclin D1 | Promotes G1 to S progression | Overexpression in breast, lung cancers | Predictive for CDK4/6 inhibitor efficacy |
| CDK4/6 | Partner with Cyclin D for G1 progression | Amplified in glioblastoma, melanoma | Targeted by palbociclib, ribociclib |
| p16 (CDKN2A) | Inhibits CDK4/6 activity | Frequently deleted in pancreatic cancer | Early diagnostic marker |
| Chk1/Chk2 | Checkpoint response to DNA damage | Inactivation leads to genomic instability | Targeted by checkpoint kinase inhibitors |
DNA Damage Response and Its Role in Cell Cycle Arrest
DNA damage, whether from radiation, chemicals, or replication errors, activates a sophisticated response system to prevent the proliferation of faulty cells. This is known as the DNA Damage Response (DDR), a critical fail-safe mechanism that intersects with the cell cycle.
The DDR primarily activates two kinases: ATM and ATR. These in turn phosphorylate downstream effectors like p53 and CHK1/CHK2, which halt the cycle at G1/S, intra-S, or G2/M checkpoints. If the damage is irreparable, apoptosis is initiated to eliminate the compromised cell.
Cancer cells often have defective DDR pathways. A common example is mutation in TP53, disabling G1 arrest, allowing cells with broken DNA to divide unchecked. This deficiency is exploited in cancer therapy, where DNA-damaging agents are more lethal to cancer cells lacking proper repair responses. Additionally, understanding how cancers escape DDR also provides insight into aggressive behavior, such as that seen in high alkaline phosphatase cancer.
Apoptosis and Cell Cycle Regulation in Cancer
Apoptosis, or programmed cell death, is intricately linked with the cell cycle. When checkpoints detect irreparable errors, signals are sent to initiate cell death through mitochondrial pathways. Key players include p53, Bax, Bcl-2, cytochrome c, and caspases.
In healthy tissues, this process prevents the propagation of damaged or abnormal cells. In cancer, however, mutations in apoptosis-regulating genes (like BCL2 overexpression or loss of BAX) allow cells to evade death despite significant abnormalities. This is one reason why cancers can accumulate vast genetic damage.
The therapeutic challenge is restoring or triggering apoptosis in resistant cancer cells. Several drugs, including BH3-mimetics, are designed to antagonize anti-apoptotic proteins, pushing malignant cells back toward programmed death, ideally after a checkpoint has failed.
Therapeutic Targeting of Cell Cycle Pathways
Modern oncology increasingly focuses on cell cycle-specific targets. This strategy allows for more precise interventions that exploit the rapid division and weak checkpoint control in tumors.
CDK inhibitors (like palbociclib), checkpoint kinase inhibitors (like prexasertib), and DNA repair pathway modulators (like PARP inhibitors) are central to this approach. By combining these drugs with conventional chemotherapy, clinicians aim to increase tumor cell kill while sparing normal tissue.
Therapies are often tailored based on the tumor’s molecular profile. For example, cancers with RB1 loss may not respond to CDK4/6 inhibitors, while BRCA-mutated tumors may be hypersensitive to DNA repair blockers. These insights are especially useful when managing complex malignancies such as retroperitoneum cancer.
Future Directions: Precision Medicine and Cell Cycle Analysis
The future of cancer treatment lies in the fusion of genomic data with functional cell cycle analysis. Using high-throughput sequencing and real-time cell imaging, researchers can determine which phase a tumor cell is vulnerable in, and which pathways are altered.
One frontier involves liquid biopsy techniques that detect circulating tumor DNA fragments indicating active mitotic activity. Others include AI-based models that predict resistance to cell cycle drugs based on mutation signatures.
Another promising area is targeting the interface between the immune system and the cell cycle. For instance, immunotherapies are now being evaluated for synergy with CDK inhibitors, as cell cycle arrest can sometimes enhance antigen presentation in tumor cells.
Furthermore, cross-analysis with other cellular diseases, like hormone-induced growth disorders or rare glandular tumors.
FAQ — Common Questions About the Eukaryotic Cell Cycle and Cancer
What is the eukaryotic cell cycle and why is it important?
The eukaryotic cell cycle is a series of regulated stages that a cell goes through to grow and divide. It includes phases like G1, S, G2, and M, and ensures that cells replicate accurately. Proper regulation of this cycle is essential to prevent mutations and cancer development.
How does the cell cycle become disrupted in cancer?
In cancer, mutations in genes that control the cell cycle—such as TP53, RB1, or CDK inhibitors—allow cells to bypass checkpoints and divide uncontrollably. This unregulated growth is a hallmark of malignancy.
What role does p53 play in the cell cycle?
p53 is a tumor suppressor protein that acts as a checkpoint regulator. It halts the cell cycle when DNA damage is detected, allowing repair or initiating apoptosis. Loss of p53 function is common in many cancers.
Are there treatments that specifically target the cell cycle?
Yes, drugs like CDK inhibitors are designed to target specific phases of the cell cycle, particularly in cancers that show dependency on these pathways. These treatments are part of a precision medicine approach.
How does apoptosis relate to cancer cell survival?
Apoptosis removes damaged or abnormal cells. Cancer cells often develop mechanisms to evade apoptosis, allowing them to survive and proliferate despite serious genetic damage.
Can early detection of cell cycle changes prevent cancer?
Detecting abnormal cell cycle activity can help identify precancerous changes. Techniques like flow cytometry or biomarkers of mitotic activity may aid early diagnosis and intervention.
What is the difference between a checkpoint and a phase in the cell cycle?
Phases (like G1 or M) are stages in the cell’s life. Checkpoints are control mechanisms within these phases that ensure all conditions are correct before progressing. Cancer often involves mutations that disable checkpoints.
How does chemotherapy interact with the cell cycle?
Many chemotherapeutic drugs target dividing cells, especially in S or M phase. This approach exploits the rapid division rate of cancer cells, though it can affect normal proliferating cells too.
What are CDKs and how are they involved in cancer?
Cyclin-dependent kinases (CDKs) are enzymes that regulate cell cycle transitions. Overactive CDKs, often due to loss of inhibitors like p16, can lead to uncontrolled proliferation—a hallmark of cancer.
Can immune therapy affect the cell cycle in cancer cells?
Emerging research suggests that immune checkpoint inhibitors may alter the tumor microenvironment in ways that interact with the cell cycle. Combination therapies with cell cycle inhibitors are under investigation.
Why is G1/S checkpoint especially important in cancer?
The G1/S transition is where the cell commits to DNA replication. It’s a critical decision point, and mutations here (e.g., in RB1 or cyclin D pathways) often lead to unchecked cell division.
How do cancer cells escape cell cycle arrest?
Through mutations, cancer cells deactivate checkpoint proteins, overexpress cyclins, or upregulate repair bypass mechanisms, allowing them to proceed even with severe genomic instability.
Is cell cycle analysis used in cancer diagnosis?
Yes, especially in hematological malignancies. Flow cytometry can identify abnormal proliferation patterns, and Ki-67 staining is commonly used to assess tumor cell proliferation.
Can cancer treatments restore normal cell cycle function?
While complete restoration is rare, targeted therapies can re-establish some control by inhibiting key overactive molecules or reinforcing suppressed pathways like p53 or p21.
Are all rapidly dividing cells cancerous?
No, some normal tissues like bone marrow, gut lining, and hair follicles also have rapid turnover. However, unregulated and context-independent proliferation is what characterizes cancer.