Seizures in Glioblastoma Patients
Seizures in Glioblastoma Patients: Understanding Causes, Mechanisms, and Relief Strategies
- Why Seizures Happen in People with Glioblastoma
- What Triggers Brain Seizures in Glioblastoma?
- How Common Are Seizures in Glioblastoma Patients?
- Underlying Causes: Why Seizures Emerge with Glioblastoma or Its Therapy
- When Are Seizures in Glioblastoma an Emergency?
- How Are Seizures Diagnosed in Patients with Glioblastoma?
- How Seizures in Glioblastoma Are Treated
- Can Seizures in Glioblastoma Be Prevented?
- Do Seizures Go Away After Glioblastoma Treatment?
- What Experts Say About Seizure Management in Glioblastoma
- Important Questions to Ask Your Doctor
- 15+ FAQ: Seizures in Glioblastoma Patients
Why Seizures Happen in People with Glioblastoma
Seizures are among the most common and often alarming symptoms experienced by patients with glioblastoma, an aggressive form of brain cancer. These episodes result from abnormal electrical activity in the brain and may be the first noticeable sign of the tumor—sometimes occurring even before a diagnosis is made.
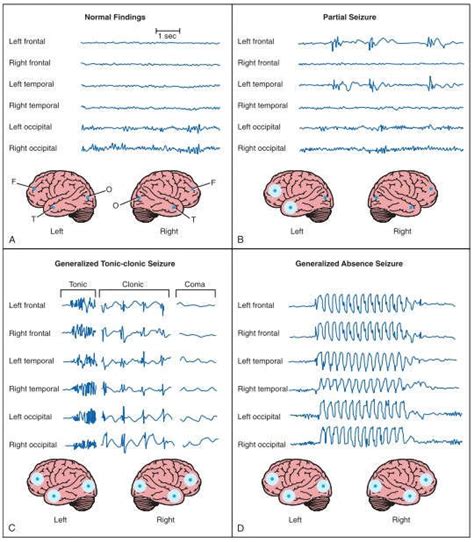
In glioblastoma, seizures can present in various forms. Some patients experience generalized tonic-clonic seizures, while others have focal seizures that manifest as twitching, sensory changes, or sudden confusion. The type and frequency of seizures often depend on the tumor’s location and proximity to functional brain tissue.
Because glioblastoma infiltrates brain parenchyma and disrupts normal neuron behavior, the brain becomes more electrically unstable. Seizures may increase with tumor progression, surgical trauma, or inflammation around the mass. Understanding these symptoms is critical for timely diagnosis and safety planning.
What Triggers Brain Seizures in Glioblastoma?
The brain’s neurons communicate via electrical impulses. Glioblastoma disrupts this system by invading brain tissue, causing inflammation, altering chemical gradients, and creating scar-like regions that become seizure foci.
Pathophysiological Mechanisms Behind Seizures in Glioblastoma
| Trigger Mechanism | How It Promotes Seizures |
| Tumor Infiltration of Cortex | Disrupts normal electrical transmission |
| Edema and Inflammation | Alters ion channel function and increases excitability |
| Blood-Brain Barrier Breakdown | Allows neurotoxic substances to enter brain tissue |
| Post-Surgical Scarring | Creates zones of hyperexcitability |
| Glioblastoma Metabolism | Alters ATP and glutamate balance, reducing neuronal stability |
The tumor’s interference with energy pathways ties closely to The Metabolic Approach to Cancer, which emphasizes how changes in mitochondrial function and glucose metabolism may trigger seizures even without visible structural damage.
How Common Are Seizures in Glioblastoma Patients?
Seizures occur in a significant percentage of glioblastoma patients—both before and after diagnosis. Studies show that epilepsy is often an early sign in low-grade gliomas, but also remains highly prevalent in glioblastoma, particularly when tumors are cortical or temporal.
Prevalence by Tumor Location and Disease Stage
| Tumor Location or Status | Likelihood of Seizures |
| Cortical (frontal/temporal) tumors | 60–75% |
| Subcortical or deep-seated tumors | ~30–40% |
| Before initial diagnosis | ~20–30% |
| During treatment (surgery/chemo) | ~50% |
| After recurrence | ~60–80% |
Seizure frequency also varies by age, tumor genetics, and metabolic features. For instance, tumors with IDH mutations are more prone to seizure activity due to altered glutamate handling—something also explored in The Eukaryotic Cell Cycle and Cancer research.
Underlying Causes: Why Seizures Emerge with Glioblastoma or Its Therapy
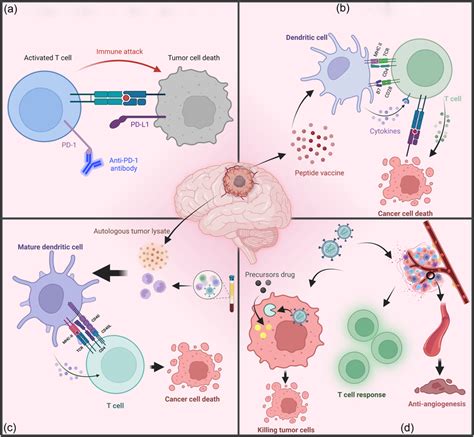
Seizures in glioblastoma are rarely caused by a single factor. Instead, they result from a complex interplay between tumor biology, surrounding inflammation, and therapy-induced tissue changes.
Overview of Contributing Factors
| Cause Type | Explanation |
| Tumor-Related | Mass effect, cortical infiltration, hemorrhage |
| Post-Surgical | Scarring, microhemorrhages, edema from resection |
| Medication-Induced | Chemo- or radiotherapy causing necrosis or neurotoxicity |
| Metabolic Imbalance | Electrolyte shifts, mitochondrial dysfunction |
| Recurrence or Progression | New tumor growth disturbing previously stable areas |
In many cases, seizure onset is a signal that the tumor is growing or affecting new parts of the brain. These episodes may also hint at poor metabolic control—one of the core themes in The Metabolic Approach to Cancer.
When Are Seizures in Glioblastoma an Emergency?
While some seizures can be brief and self-limited, others may become dangerous, even life-threatening. In glioblastoma patients, any new seizure—or change in an existing pattern—requires immediate medical attention.
Particularly alarming signs include seizures lasting longer than five minutes (status epilepticus), multiple seizures without full recovery in between, loss of consciousness, difficulty breathing, or abnormal movements involving the entire body. These can indicate a serious neurological crisis, rapid tumor progression, or intracranial pressure changes.
In addition, a seizure that presents with sudden personality change, slurred speech, or weakness on one side may suggest a stroke or hemorrhage within the tumor—a known complication in high-grade cancer.
Emergency Seizure Indicators in Glioblastoma
| Symptom or Event | Possible Emergency Cause |
| Seizure lasting >5 minutes | Status epilepticus |
| New onset confusion, agitation, or coma | Raised intracranial pressure or mass effect |
| Focal weakness or paralysis post-seizure | Postictal paresis or tumor expansion |
| Uncontrolled muscle jerks + unconsciousness | Generalized tonic-clonic seizure |
| Recurrent seizures in short succession | Progression or edema-related instability |
These situations demand hospitalization, neuroimaging, and potentially ICU-level seizure management.
How Are Seizures Diagnosed in Patients with Glioblastoma?
Diagnosing the cause and type of seizure in glioblastoma patients is a multidimensional process involving neuroimaging, clinical history, and functional tests. Since seizures may mimic other neurological issues such as transient ischemic attacks (TIAs) or tumor-related symptoms, precision is key.
Common Diagnostic Methods in Glioblastoma-Related Seizures
| Tool or Procedure | Clinical Purpose |
| MRI Brain with Contrast | Detects tumor location, edema, bleeding, and recurrence |
| EEG (Electroencephalogram) | Identifies seizure type and focal zones of abnormal activity |
| CT Scan | Used in emergency settings to assess for hemorrhage or hydrocephalus |
| Metabolic Panel | Checks for electrolyte imbalances or medication toxicity |
| Neurological Examination | Evaluates deficits post-seizure or aura features |
| Medication Blood Levels | Assesses antiepileptic drug (AED) efficacy or toxicity |
In postoperative or radiated brains, distinguishing seizure activity from The Eukaryotic Cell Cycle and Cancer–related changes (e.g., pseudoprogression) is especially challenging, which is why advanced imaging and EEG correlation are often needed.
How Seizures in Glioblastoma Are Treated
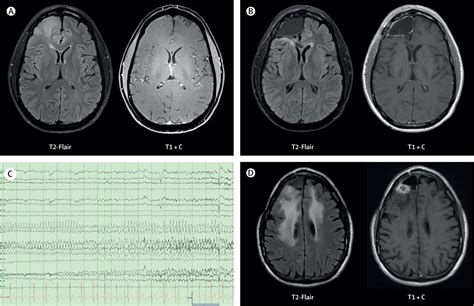
Seizure control in glioblastoma is an integral part of the treatment plan and quality-of-life management. Therapy includes acute stabilization, long-term medication use, and sometimes neurosurgical adjustments.
Clinical Management of Seizures
| Treatment Approach | Application |
| Antiepileptic Drugs (AEDs) | First-line therapy for seizure prevention and control (e.g., levetiracetam, lacosamide) |
| Corticosteroids (e.g., dexamethasone) | Reduces brain edema and inflammation contributing to seizures |
| Tumor-Directed Therapies | Chemotherapy, radiation, or surgical debulking to reduce tumor-related irritability |
| Resective Surgery | In selected patients, seizure focus and tumor may be removed together |
| Neurostimulation Devices | Vagus nerve stimulators or responsive neurostimulation for drug-resistant cases |
| Ketogenic Diet (experimental) | Sometimes explored based on insights from The Metabolic Approach to Cancer |
AEDs are usually started even after a single seizure in glioblastoma patients, given the high risk of recurrence. Drug selection often favors those with minimal interactions with chemotherapy or cognitive side effects.
Can Seizures in Glioblastoma Be Prevented?
Preventing seizures entirely in glioblastoma is difficult, but proactive strategies can reduce frequency and severity. Early initiation of antiepileptic drugs in high-risk patients—such as those with cortical tumors—may delay onset.
Moreover, optimizing tumor control through maximal safe resection and targeted therapies can reduce irritation of surrounding brain tissue. Managing metabolic contributors like glucose fluctuations, dehydration, or sleep disruption also supports brain stability.
Preventive Strategies for Seizure Control
| Strategy | Benefit |
| Prophylactic AEDs in high-risk tumors | Reduces early postoperative seizure risk |
| Consistent corticosteroid use (tapered properly) | Controls edema and inflammation |
| Regular imaging and tumor monitoring | Detects progression before symptoms arise |
| Metabolic support (diet, hydration) | May stabilize neuronal function |
| Avoidance of triggers (stress, lights, fatigue) | Lowers provocation threshold |
Some neuro-oncologists incorporate supportive interventions from The Metabolic Approach to Cancer (e.g., low-glycemic diets or mitochondrial support), although these are still under clinical investigation and not universally applied.
Do Seizures Go Away After Glioblastoma Treatment?
Seizure activity may decrease after initial glioblastoma treatment, but complete resolution is not guaranteed. Tumor resection, radiation, and chemotherapy often reduce the tumor burden, thereby decreasing seizure frequency. However, if the seizure focus remains in damaged or scarred brain tissue, some level of epileptic activity may persist.
Patients who respond well to surgery—especially those with tumors in non-eloquent cortical areas—often report a significant drop in seizures or even full remission. But for many, seizures become a chronic symptom, requiring long-term antiepileptic therapy.
Prognosis for Seizure Control
| Post-Treatment Scenario | Likelihood of Seizure Control |
| Complete resection, no residual edema | High chance of seizure freedom |
| Partial resection with cortical involvement | Moderate seizure reduction, long-term AED use |
| Tumor recurrence or progression | Seizure recurrence likely |
| Radiation necrosis or gliosis post-therapy | Risk of chronic focal epilepsy |
As glioblastoma is an aggressive cancer, seizure patterns may also reflect disease activity. A sudden return of seizures in a previously stable patient can signal recurrence or treatment failure.
What Experts Say About Seizure Management in Glioblastoma
Neuro-oncology specialists emphasize a proactive and individualized approach to seizure control. While seizure prevention isn’t always feasible, improving quality of life is achievable with early intervention and multidisciplinary management.
Experts recommend that all patients with cortical or temporal glioblastomas undergo routine seizure screening and receive AEDs when indicated. Additionally, steroids like dexamethasone are often used perioperatively to reduce swelling and lower seizure risk.
There is growing interest in integrating metabolic interventions—such as ketogenic dietary strategies—as adjuncts, based on evolving understanding from The Metabolic Approach to Cancer. These approaches aim to reduce seizure threshold and potentially slow tumor progression through altered cellular energy metabolism.
Neurosurgeons stress the importance of maximizing tumor resection in seizure-prone areas, provided that critical functions are preserved. Meanwhile, epileptologists collaborate to fine-tune AED regimens and assess whether implantable devices may benefit those with drug-resistant seizures.
Important Questions to Ask Your Doctor
Effective communication is vital in managing seizures alongside glioblastoma treatment. Patients and caregivers should feel empowered to ask targeted questions that inform both daily management and long-term planning.
| Question | Why It Matters |
| What kind of seizures am I experiencing? | Helps with diagnosis and medication choice |
| Are my seizures caused by the tumor or treatment? | Clarifies origin and necessary interventions |
| What are the best medications for my case? | Personalizes therapy based on side effect profile |
| How long will I need to take anti-seizure drugs? | Sets realistic expectations |
| Can seizures return even after surgery? | Prepares for recurrence risk |
| Should I keep a seizure diary? | Aids in tracking and medication adjustment |
| What should my family do if I have a seizure? | Provides safety instructions |
| Are there warning signs before a seizure? | Improves self-monitoring and response |
| Can I drive with this diagnosis? | Addresses legal and safety concerns |
| Will treatment reduce seizure activity over time? | Offers reassurance or alternative planning |
| Do I need to avoid certain activities or stimuli? | Reduces risk of provoked episodes |
| What imaging or tests will track this symptom? | Guides future monitoring |
| Is there a role for surgery or neurostimulation? | Explores advanced options for drug resistance |
| How does this affect my quality of life and prognosis? | Provides honest context |
| Can metabolic or dietary changes help? | Opens discussion about The Metabolic Approach to Cancer or experimental options |
15+ FAQ: Seizures in Glioblastoma Patients
1. Can seizures be the first symptom of glioblastoma?
Yes. In up to 30% of patients, the first sign of glioblastoma is a seizure, especially when the tumor is located near the cortex.
2. What do glioblastoma-related seizures look like?
They can vary—some are generalized with convulsions, others are focal, causing twitching, confusion, or unusual sensations.
3. How are these seizures different from those in epilepsy?
They are secondary to tumor activity and inflammation, and may not follow typical epilepsy patterns. They also carry higher risks due to the tumor’s location and progression.
4. Are seizures in glioblastoma treatable?
Yes, with AEDs, surgery, and supportive therapies. Many patients achieve control, though some experience chronic or recurring symptoms.
5. Will surgery stop the seizures completely?
Sometimes. If the seizure focus is resected during tumor removal, patients may become seizure-free, but this depends on tumor location and brain integrity.
6. Is radiation therapy likely to cause seizures?
Radiation may cause brain swelling or necrosis, which can provoke seizures—especially weeks or months after treatment.
7. Do all patients with glioblastoma have seizures?
No. Around 50–70% experience seizures at some point, but not all patients do.
8. Should everyone with glioblastoma be on anti-seizure meds?
Not always. Prophylactic AED use is typically reserved for high-risk cases or post-operative periods.
9. Can a seizure mean my tumor is growing again?
It could. New or worsening seizures often prompt re-imaging to check for recurrence.
10. Are seizures painful?
Not during the event (most patients are unconscious), but postictal symptoms—like headache, confusion, and muscle soreness—can be distressing.
11. What happens during a focal seizure?
Patients may experience sensations like déjà vu, tingling, speech difficulty, or visual disturbances without losing consciousness.
12. Can seizures be fatal in glioblastoma?
Rarely. However, status epilepticus or trauma from seizures can lead to life-threatening complications.
13. Will I need to go to the hospital after every seizure?
Not necessarily. If seizures are brief and known, outpatient management may be sufficient. Emergencies require ER care.
14. Do seizures always mean treatment isn’t working?
Not always. Some occur due to inflammation or scarring—not active tumor growth.
15. Can lifestyle changes reduce seizure risk?
Yes—adequate sleep, stress reduction, medication compliance, and avoiding seizure triggers are all helpful. Some patients explore The Metabolic Approach to Cancer with medical guidance.