PTP1B Role in Breast Cancer: Insights and Research Findings
- Understanding PTP1B: The Basics
- How PTP1B Functions in Normal and Cancerous Cells
- PTP1B Expression in Breast Cancer Subtypes
- Why PTP1B Can Be a Therapeutic Target
- PTP1B and HER2-Positive Breast Cancer: A Molecular Partnership
- Comparing PTP1B Roles in Normal Cells vs. Breast Cancer Cells
- PTP1B in Metastasis and Tumor Invasion
- PTP1B and the Tumor Microenvironment
- PTP1B and Hormone Receptor-Positive Breast Cancer
- Genetic Regulation of PTP1B: What Controls Its Activity?
- Challenges in Targeting PTP1B Clinically
- PTP1B and Resistance to Existing Therapies
- Preclinical Studies on PTP1B Inhibitors
- Potential Biomarkers for PTP1B-Targeted Therapy
- Future Directions in Research and Clinical Application
- What Patients and Providers Should Know About PTP1B
- FAQ: What is PTP1B in Breast Cancer?
Understanding PTP1B: The Basics
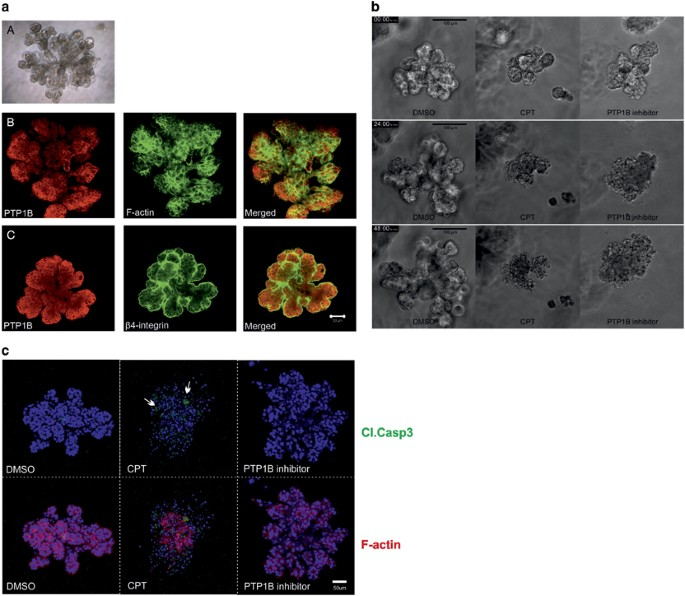
PTP1B stands for Protein Tyrosine Phosphatase 1B, a regulatory enzyme that plays a central role in cellular signaling. It acts by removing phosphate groups from tyrosine residues on proteins, thereby modulating various signaling pathways. Under normal conditions, PTP1B helps maintain the balance between cell growth and cell inhibition by downregulating certain tyrosine kinase signals.
PTP1B is encoded by the PTPN1 gene and is widely expressed in metabolic tissues, the immune system, and many epithelial cells. In the context of cancer, it was originally thought to act as a tumor suppressor because it negatively regulates growth factor signaling. However, more recent research reveals a dual nature: PTP1B can act as a promoter of tumor progression under specific oncogenic contexts, including in breast cancer.
This paradox—where a molecule involved in negative regulation can also fuel tumorigenesis—is now the subject of intense investigation, especially in HER2-positive and triple-negative breast cancer models.
How PTP1B Functions in Normal and Cancerous Cells
In healthy cells, PTP1B serves as a molecular “brake” that dampens hyperactive growth signals. It dephosphorylates key proteins in pathways like insulin signaling, leptin regulation, and the JAK/STAT cascade. This role is crucial for controlling cell proliferation, differentiation, and metabolic balance.
In breast cancer, however, PTP1B may lose this regulatory control or be hijacked by oncogenic systems. For example, in HER2-positive tumors, PTP1B appears to cooperate with HER2 to promote tumorigenesis. Rather than suppressing growth, PTP1B may support oncogenic survival signals, facilitate evasion of apoptosis, and influence metastatic potential.
This context-specific behavior has been shown in genetically modified mouse models, where deletion of PTP1B can prevent HER2-driven tumor formation. Conversely, overexpression of PTP1B in mammary epithelial cells leads to increased tumor incidence.
Understanding this dual functionality is key to designing therapies that can selectively target PTP1B without disrupting its beneficial roles in other tissues.
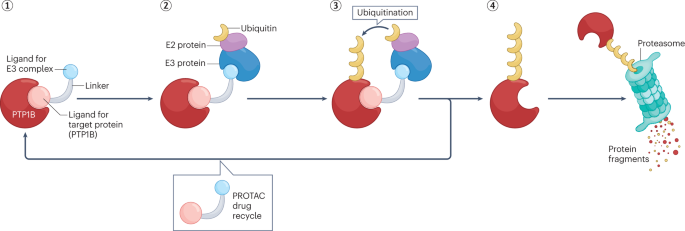
PTP1B Expression in Breast Cancer Subtypes
PTP1B expression is not uniform across all breast cancer subtypes. Its expression is elevated in HER2-positive breast cancers and, to a lesser extent, in triple-negative tumors. Luminal (ER-positive) cancers tend to show lower or variable levels of PTP1B activity, though its functional role in these subtypes remains under exploration.
High PTP1B levels have been correlated with poor prognosis in certain breast cancer cohorts, especially when co-expressed with HER2. The protein has also been detected in the tumor stroma and associated immune cells, suggesting a role beyond the cancerous epithelium itself.
Recent studies using immunohistochemistry and RNA sequencing data have helped map PTP1B activity across large tumor databases. These findings indicate that PTP1B may serve not only as a therapeutic target but also as a potential prognostic biomarker in certain patients.
As with many cancer-related enzymes, its behavior reflects the cellular context and microenvironment—similar to how inflammation and molecular variation affect diseases like those discussed in colorectal cancer vs IBS, where shared symptoms belie radically different cellular processes.
Why PTP1B Can Be a Therapeutic Target
The realization that PTP1B can promote tumor progression in certain settings has spurred efforts to develop targeted inhibitors. Small molecule inhibitors of PTP1B have been studied in diabetes and obesity research for over a decade, given its role in insulin signaling. More recently, these compounds are being repurposed or redesigned for oncology.
Targeting PTP1B in breast cancer is appealing because of its specific involvement in HER2 signaling and metastasis-related pathways. Animal models have shown that pharmacologic inhibition of PTP1B can reduce tumor volume, decrease lung metastasis, and enhance sensitivity to trastuzumab (Herceptin), a HER2-targeted therapy.
However, systemic inhibition of PTP1B carries risks—particularly in metabolic and immune systems where the enzyme has protective roles. Researchers are now investigating ways to develop tissue-specific or context-dependent inhibitors to minimize side effects.
Understanding how PTP1B is activated, localized, and regulated will be essential to this effort. These insights may also contribute to broader knowledge about context-driven carcinogenesis, such as in stage 4 neuroendocrine cancer spread to liver life expectancy, where metastatic behavior varies drastically by biological profile.
PTP1B and HER2-Positive Breast Cancer: A Molecular Partnership
HER2-positive breast cancer is driven by amplification of the HER2/neu gene, resulting in overexpression of the HER2 receptor—a protein that promotes cell proliferation and survival. PTP1B, surprisingly, has been found to support this signaling pathway rather than inhibit it. In multiple preclinical studies, PTP1B appears to enhance HER2-driven tumor progression by modulating downstream components like Src and PI3K.
In mouse models engineered to express both HER2 and PTP1B in mammary tissue, tumors developed more rapidly and showed greater invasiveness. When PTP1B was genetically deleted or pharmacologically inhibited, tumor incidence and growth rates were significantly reduced.
This synergy suggests that PTP1B acts as a signal amplifier within the HER2 pathway, possibly by regulating phosphatase-sensitive feedback loops. These findings are significant because they provide a rationale for dual targeting strategies: combining HER2 blockade (e.g., trastuzumab) with PTP1B inhibition could theoretically enhance treatment response and delay resistance.
While clinical trials are still pending, this line of research offers promise—especially for patients with aggressive HER2-positive disease and limited response to standard regimens.
Comparing PTP1B Roles in Normal Cells vs. Breast Cancer Cells
| Aspect | Normal Cells | Breast Cancer Cells (HER2-positive focus) |
| Primary Role | Regulation of insulin signaling, growth factor attenuation | Support of oncogenic signaling, HER2 amplification loop |
| Localization | Endoplasmic reticulum membrane, cytoplasm | Cytoplasm, tumor cell membranes, sometimes tumor stroma |
| Impact on Proliferation | Inhibits excessive growth | Enhances cell survival, promotes proliferation |
| Effect on Apoptosis | Neutral or supportive | Inhibits apoptosis in cancerous context |
| Therapeutic Relevance | Metabolic disorders (e.g., diabetes) | Target for anti-HER2 and anti-metastatic strategies |
PTP1B in Metastasis and Tumor Invasion
Beyond its role in primary tumor growth, PTP1B is emerging as a key player in metastasis—the process by which cancer spreads to distant organs. In breast cancer, PTP1B has been implicated in several steps of this cascade: from local tissue invasion and epithelial-mesenchymal transition (EMT) to survival in the bloodstream and colonization of distant tissues.
One proposed mechanism involves PTP1B’s interaction with integrins and focal adhesion kinases (FAKs), which are essential for cell adhesion and movement. By modulating phosphorylation at these points, PTP1B may enable cancer cells to detach from their original site and migrate through the extracellular matrix.
Additionally, PTP1B may influence immune cell infiltration into the tumor microenvironment. In preclinical models, tumors with high PTP1B activity showed fewer cytotoxic T-cells and more immunosuppressive macrophages. This immunomodulatory role is still under active investigation but raises the possibility that PTP1B contributes to immune evasion—a hallmark of metastatic competence.
Ultimately, understanding how PTP1B facilitates metastasis may help explain why some breast cancers are more aggressive than others, even within the same molecular subtype.
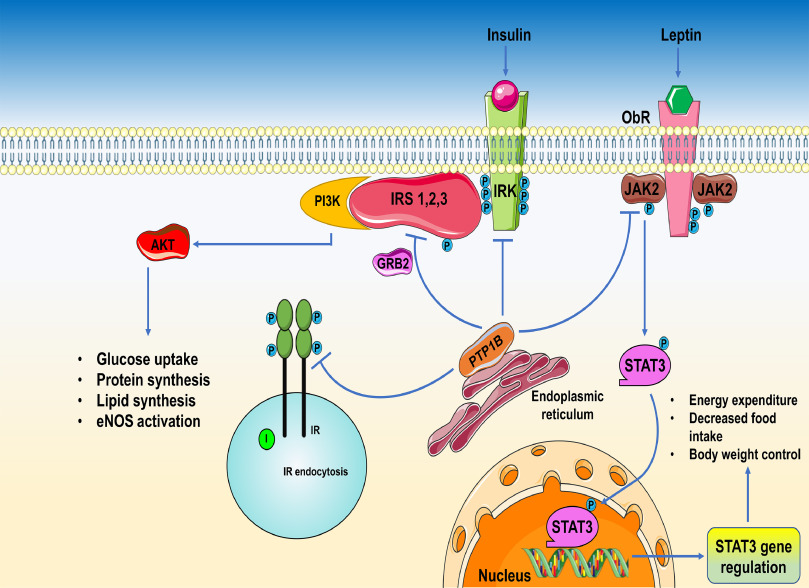
PTP1B and the Tumor Microenvironment
PTP1B’s influence extends beyond the cancer cell itself and into the tumor microenvironment (TME)—a dynamic ecosystem composed of immune cells, fibroblasts, blood vessels, and extracellular matrix. Evidence suggests that PTP1B affects stromal remodeling, angiogenesis, and immune response within the TME.
For example, endothelial cells lining tumor-associated blood vessels express PTP1B, which may regulate vascular permeability and angiogenic signaling. In mouse models, inhibition of PTP1B not only reduced tumor volume but also normalized blood vessel structure, enhancing chemotherapy delivery.
Fibroblasts within the TME can also express PTP1B. These cells play a critical role in matrix deposition and paracrine signaling, affecting both tumor stiffness and access to nutrients. Elevated PTP1B in cancer-associated fibroblasts has been linked to more desmoplastic (fibrous) tumors, which are harder to treat.
Finally, PTP1B may modulate cytokine release and immune cell recruitment. This makes it a potential target for combinatorial therapies that aim not just to kill tumor cells, but to “reprogram” the tumor environment into a less supportive space for cancer growth.
This interaction between a seemingly internal molecular regulator and its external surroundings parallels concepts explored in does polyester cause cancer, where chronic environmental contact alters cellular behavior over time.
PTP1B and Hormone Receptor-Positive Breast Cancer
While PTP1B is best known for its role in HER2-positive breast cancer, its function in hormone receptor-positive (HR+) disease is less clearly defined. HR+ breast cancers, which express estrogen or progesterone receptors, generally follow a slower growth pattern and respond well to endocrine therapies like tamoxifen or aromatase inhibitors.
Initial studies suggested that PTP1B may be less relevant in ER-positive tumors. However, more recent findings indicate that PTP1B can still modulate downstream signaling from estrogen receptors through interaction with other growth factor pathways, such as insulin-like growth factor (IGF-1) and epidermal growth factor (EGF).
In some cases, PTP1B overexpression may contribute to resistance against hormonal therapy by enhancing survival signals that bypass estrogen dependence. This makes it a potential co-target in combination with anti-estrogen therapy, especially in cases where endocrine resistance is observed.
Further research is needed to define whether PTP1B is a true therapeutic liability in HR+ tumors or a secondary player influenced by other dominant pathways.
Genetic Regulation of PTP1B: What Controls Its Activity?
The activity of PTP1B is not solely determined by its protein levels. Multiple layers of regulation affect its function, starting at the genomic level with the PTPN1 gene. Promoter methylation, transcription factor binding, and chromatin remodeling all influence how much PTP1B mRNA is produced in any given cell.
Post-transcriptional modifications also play a major role. These include phosphorylation, oxidation (which temporarily inactivates the enzyme), and interaction with regulatory subunits. PTP1B is localized on the cytoplasmic side of the endoplasmic reticulum, which also affects its access to substrates.
In tumors, altered regulation may result in increased PTP1B activity despite normal expression levels. This can occur through inflammatory cytokines or oncogenic mutations that stabilize the enzyme or amplify its access to targets. Understanding these mechanisms is key for developing targeted drugs that inhibit not just expression, but enzymatic function.
Tumor heterogeneity means that in some cancers, PTP1B behaves differently depending on the surrounding genomic and epigenetic context. This complexity is part of the challenge in designing truly selective PTP1B inhibitors.
Challenges in Targeting PTP1B Clinically
Despite the strong rationale, developing safe and effective PTP1B inhibitors has proven challenging. First-generation compounds had poor bioavailability and off-target effects, limiting their use in clinical settings. Because PTP1B shares structural homology with other phosphatases, especially TC-PTP, achieving selectivity without toxicity is technically difficult.
Another barrier is tissue specificity. PTP1B is widely expressed in the liver, brain, and adipose tissue—meaning systemic inhibition could disrupt glucose regulation, neurobiology, or lipid metabolism. For breast cancer, where local tumor inhibition is preferred, researchers are now focusing on delivery mechanisms that limit exposure to non-target organs.
Encapsulation in nanoparticles, tumor-specific antibody conjugates, and prodrug forms are all being explored to refine drug delivery. Biomarker-driven patient selection will also be important, identifying those whose tumors rely most heavily on PTP1B for survival or metastasis.
These challenges reflect broader difficulties in precision oncology: balancing efficacy with tolerability, and targeting molecular mechanisms without disrupting essential physiology.
PTP1B and Resistance to Existing Therapies
One of the most clinically relevant aspects of PTP1B is its emerging role in resistance to established breast cancer treatments. In HER2-positive tumors, for example, PTP1B may facilitate escape from trastuzumab-induced growth arrest by reactivating downstream pathways like PI3K/AKT or SRC.
In hormone receptor-positive cases, PTP1B overexpression has been associated with poor response to aromatase inhibitors, possibly by maintaining survival signals through cross-talk with insulin or EGF receptors. This suggests that dual targeting of PTP1B and these pathways may help overcome treatment resistance in otherwise refractory tumors.
Mechanistically, PTP1B appears to function as a molecular switch that allows cancer cells to bypass upstream inhibition by restoring downstream signaling flux. This ability makes it a critical node in resistance circuitry—a pattern also observed in other tumors with treatment failure patterns, such as those discussed in stage 4 neuroendocrine cancer spread to liver life expectancy, where resistance is linked to microenvironment and molecular redundancy.
Preclinical Studies on PTP1B Inhibitors
Most of our current knowledge about PTP1B inhibitors in cancer comes from preclinical work using cell lines and animal models. In vitro studies show that selective inhibition of PTP1B in HER2-positive breast cancer cells leads to reduced proliferation, increased apoptosis, and decreased cell motility. These effects are amplified when combined with other agents such as trastuzumab or PI3K inhibitors.
In vivo models support these findings. Mice treated with PTP1B inhibitors show slower tumor growth, reduced angiogenesis, and in some cases, delayed metastasis formation. Pharmacodynamic markers such as decreased phospho-SRC and increased cleaved caspase-3 confirm that these inhibitors affect both survival and invasion pathways.
Importantly, PTP1B inhibition does not appear to harm normal breast tissue in these models, suggesting a therapeutic window exists. However, effects on glucose homeostasis and body weight in some animal studies highlight the need for precise targeting to avoid systemic side effects.
These promising results justify the movement toward early-phase clinical trials. Yet much work remains in optimizing drug design, delivery, and patient selection strategies.
Potential Biomarkers for PTP1B-Targeted Therapy
To successfully implement PTP1B inhibition in clinical settings, oncologists must first identify which patients are most likely to benefit. This requires reliable biomarkers that indicate dependence on PTP1B signaling.
Candidate biomarkers include high PTP1B protein expression (measurable by immunohistochemistry), PTPN1 mRNA levels, and co-expression of HER2. Tumors with elevated phospho-SRC or high PI3K pathway activity may also be more sensitive to PTP1B blockade.
Genomic features such as copy number amplification of the PTPN1 gene or mutations in related regulators might further refine patient selection. Liquid biopsies to track response through circulating tumor DNA or exosome content are under investigation.
Ultimately, a composite biomarker approach—combining protein levels, gene expression, and signaling signatures—will likely be necessary. This mirrors the complexity of targeting any intracellular pathway, where biology is rarely linear or isolated.
Future Directions in Research and Clinical Application
PTP1B is no longer viewed as a mere metabolic enzyme. In breast cancer, it is increasingly recognized as a signaling hub with dual roles in promoting or suppressing tumor growth depending on context. The next decade will likely bring tissue-specific inhibitors, better understanding of tumor-immune interactions, and deeper integration of PTP1B into combination therapies.
Future clinical trials will need to address not just efficacy, but tolerability and patient-reported outcomes. If PTP1B inhibitors can improve response to existing drugs or reverse resistance, they may play a key role in modern breast cancer treatment algorithms.
There is also interest in combining PTP1B targeting with immunotherapy. If the enzyme suppresses immune infiltration or function, its inhibition could potentially improve response to checkpoint blockade—an area already being explored in triple-negative breast cancer.
As breast cancer care moves toward personalization, PTP1B may evolve from a niche curiosity to a central component in precision oncology strategies.
What Patients and Providers Should Know About PTP1B
PTP1B is a complex but increasingly important molecule in breast cancer biology. Once thought to be simply a metabolic regulator, it is now understood to have direct influence on tumor growth, treatment resistance, and metastatic potential—especially in HER2-positive and hormone-resistant disease.
While not yet part of standard care, PTP1B represents a promising therapeutic target supported by robust preclinical data. Researchers are working toward clinically viable inhibitors that are safe, selective, and effective. Until then, its main role remains as a prognostic marker and experimental target.
Understanding the function of PTP1B helps providers think more broadly about how intracellular pathways interact and evolve under treatment pressure. For patients, it offers a glimpse into the cutting edge of breast cancer science—a frontier where the next generation of therapies is being shaped.
FAQ: What is PTP1B in Breast Cancer?
What does PTP1B stand for?
PTP1B stands for Protein Tyrosine Phosphatase 1B, an enzyme that regulates cellular signaling by removing phosphate groups from tyrosine residues on proteins.
How is PTP1B involved in breast cancer?
In breast cancer, especially HER2-positive cases, PTP1B can support tumor growth and survival by amplifying oncogenic signaling pathways, rather than suppressing them.
Is PTP1B always harmful in cancer?
No. PTP1B has a dual role. While it can act as a tumor suppressor in some contexts, it may promote tumor progression in others depending on molecular environment.
Why is PTP1B significant in HER2-positive breast cancer?
PTP1B enhances HER2-driven signaling, helping tumors grow faster and potentially resist therapies like trastuzumab. This makes it a valuable co-target.
Does PTP1B contribute to metastasis?
Yes. It influences cell migration, invasion, and immune evasion—key processes in cancer spread to other organs.
Is PTP1B relevant in hormone receptor-positive tumors?
Possibly. It may play a secondary role in endocrine resistance by interacting with growth factor signaling pathways.
Can PTP1B be used as a biomarker?
Yes. High expression levels of PTP1B or its gene PTPN1 may indicate poor prognosis and increased likelihood of treatment resistance.
Are there drugs that target PTP1B?
Several experimental inhibitors exist, primarily in preclinical testing. Human trials are anticipated as drug design improves.
What are the challenges in targeting PTP1B?
Achieving specificity without affecting other vital tissues like the liver and brain is a major obstacle in drug development.
How does PTP1B affect immune response?
PTP1B may reduce immune cell infiltration into tumors and shift immune profiles toward more suppressive environments.
Is PTP1B involved in resistance to chemotherapy?
Indirectly, yes. It can reactivate downstream pathways even when upstream receptors are blocked, leading to resistance.
Can inhibiting PTP1B make other treatments work better?
Preclinical data suggest that blocking PTP1B may enhance response to HER2-targeted therapies and potentially immunotherapy.
Does PTP1B affect normal breast tissue?
In healthy tissue, PTP1B helps maintain growth balance, but it does not appear to cause damage when selectively inhibited in tumors.
Is there a connection between PTP1B and other cancers?
Yes. PTP1B has been studied in colorectal, lung, and pancreatic cancers as well, showing similar dual roles in tumor biology.
What should patients know about PTP1B right now?
While not yet part of routine treatment, PTP1B is a highly promising target. It may guide future therapies and offer insights into treatment resistance and prognosis.