Plague (Yersinia pestis): An Ancient Disease in the Modern World
- 1. Introduction — Plague (Yersinia pestis): An Ancient Disease in the Modern World
- 2. Bacteriology and Pathogenesis — What Makes Yersinia pestis So Dangerous?
- 3. Epidemiology — Where Is the Plague Now, and Who’s Still at Risk?
- 4. Clinical Manifestations — What Happens When Plague Strikes the Body?
- 5. Diagnosis — How Do You Catch a Killer Before It’s Too Late?
- 6. Treatment and Management — Can Modern Medicine Beat an Ancient Killer?
- 7. Prevention and Control — How Do We Keep the Plague From Striking Back?
- 8. Recent Developments (2025–2026) — What’s New in the World of Plague?
- FAQ: Key Questions About Plague (Yersinia pestis)
- Final Thoughts
Introduction — Plague (Yersinia pestis): An Ancient Disease in the Modern World
Can a disease that devastated medieval cities still matter today? When we hear the word “plague,” most of us imagine black-robed doctors, rat-infested alleyways, and mass graves from centuries ago. But what if I told you that Yersinia pestis, the bacterium behind the Black Death, hasn’t gone anywhere? It’s not just a relic of the past—it’s still lurking in the modern world.
Why does that matter? Well, consider this: the plague hasn’t just survived history—it’s adapted to it. While outbreaks today are far rarer and usually controlled with antibiotics, they still happen. That persistent potential mirrors other cross-species threats we’ve explored—Avian Influenza H5N9, for instance, reminds us that zoonotic viruses, like bacteria, don’t need high case counts to reshape public health priorities.
Here’s another question worth pondering: if we’ve “conquered” so many infectious diseases, why do some, like the plague, keep slipping through the cracks? Is it about biology, or is it about how societies respond—or fail to respond—to emerging and re-emerging threats?
In this deep dive, we’ll explore the life and legacy of Yersinia pestis—from its genetic quirks to its devastating clinical forms. But we’ll also ask: what lessons does it teach us about public health in the 21st century? Why should a microbe from the Middle Ages still be part of our global medical conversation?
Let’s find out.
Bacteriology and Pathogenesis — What Makes Yersinia pestis So Dangerous?
So, what exactly is Yersinia pestis? We throw around the name like it’s a single villain in a medieval horror story, but the truth is a little more nuanced—and, frankly, a lot more interesting. This isn’t just any bacterium. This is a highly evolved, genetically streamlined pathogen, built not just to survive, but to hijack and destroy.
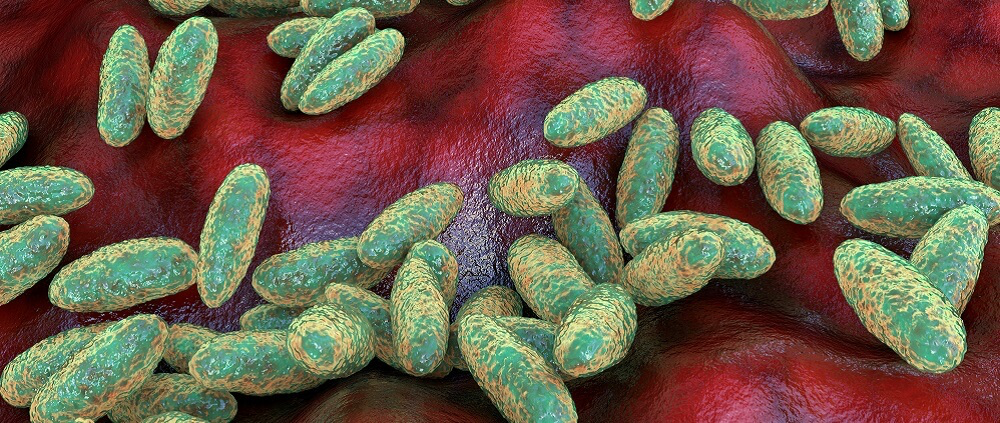
Start with the basics: it’s a gram-negative, rod-shaped bacterium, small and unassuming under a microscope. But don’t let its modest size fool you. Its genetic blueprint reads like a criminal résumé. Unlike many bacteria that wear their virulence like badges, Y. pestis is covert. It doesn’t flash its weapons—it deploys them with surgical precision.
One of the most fascinating—and troubling—features of Y. pestis is how it evolved from a relatively harmless gut bacterium, Yersinia pseudotuberculosis. That’s right: plague’s ancestor just gave people mild stomach bugs. So what changed? Over time, Y. pestis picked up genes from mobile genetic elements—plasmids—that gave it a deadly edge. With these new genetic tools, it learned how to infiltrate host defenses, shut down immune responses, and turn macrophages—the very cells meant to kill invaders—into unwitting Uber drivers straight to the lymph nodes.
And the lymph nodes? That’s plague’s playground. Once inside, Y. pestis starts replicating rapidly, forming the infamous “buboes” that give bubonic plague its name. But here’s where things get even darker: this bacterium doesn’t just grow. It disarms the immune system. It uses a Type III secretion system—like a molecular syringe—to inject toxins into host cells, paralyzing them. The body tries to mount a defense, but by then, it’s usually too late. The bacteria are already spreading, sometimes slipping into the bloodstream (septicemic plague) or even making their way to the lungs (pneumonic plague), where the infection becomes explosively contagious.
It begs the question—how does something so tiny do so much damage, and so quickly?
Maybe the real question is: how did we ever survive it in the first place?
But there’s another layer here. Modern genetic sequencing has shown that Y. pestis isn’t a static entity. It continues to mutate and adapt. It’s relatively slow compared to viruses, but it’s not frozen in time. That’s important because it means the bacterium that caused the Black Death isn’t identical to the one behind modern cases in Madagascar or the American Southwest. The essence is the same, yes—but evolution hasn’t stopped.
So we’re dealing with a shape-shifter. A microbe that once transformed from a gut passenger into a pandemic-level killer. One that continues to lurk in the wild, perfectly equipped to strike when the opportunity arises.
And that brings us to the next natural question: where, exactly, is it hiding now?
Let’s move on to epidemiology.
Epidemiology — Where Is the Plague Now, and Who’s Still at Risk?
When people hear that the plague still exists, the most common reaction is disbelief. Wait, what? I thought we got rid of that centuries ago. It’s not an unreasonable assumption. After all, with all the antibiotics, sanitation systems, and modern healthcare, how could something so ancient possibly still be in circulation?
But it is. Yersinia pestis never really left. It just got quieter.
So where is it hiding?
You’d be surprised. Plague still has strongholds in parts of Africa, Asia, and the Americas. Yes, the Americas. The western United States—places like New Mexico, Arizona, Colorado—see a handful of human cases almost every year. Not in big cities, of course, but in rural areas, especially those near rodent habitats. And Madagascar? It’s actually a global hotspot, with seasonal outbreaks and hundreds of cases reported annually.
But it’s not just about where it shows up—it’s about how it stays alive. And here’s where the ecology of plague starts to feel almost like a slow-burn thriller.
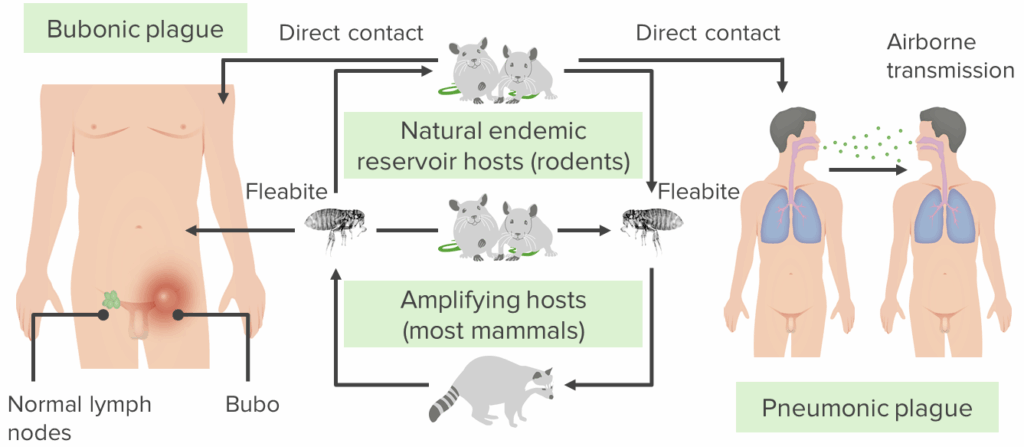
The bacterium lives in natural reservoirs, usually small mammals like ground squirrels, prairie dogs, and various species of rats. These animals serve as long-term hosts—infected but often asymptomatic. The bacterium passes between them via fleas, which act as tiny biological syringes. When an infected flea bites a rodent, the cycle continues. When the rodent dies and the flea needs a new host? That’s where humans can come into the picture.
It’s a classic case of zoonotic spillover—bacteria crossing the species barrier from animal to human. And while we often talk about zoonoses in the context of exotic viruses, Y. pestis has been doing this for thousands of years. It’s a master of the jump.
Interestingly, these dynamics echo what we’ve seen with other lesser-known pathogens like HKU5-CoV-2, a bat-origin coronavirus with potential relevance to future outbreaks. The patterns are eerily similar—natural reservoirs, subtle spillovers, and the occasional spark that can escalate quickly.
So who’s actually at risk today?
In truth, very few people—statistically speaking. We’re talking dozens to hundreds of human cases worldwide per year. But the risk isn’t uniform. Hunters, farmers, park rangers, or even campers who handle dead animals or get too close to rodent colonies—they’re far more exposed than the average city-dweller. Add in fragile healthcare systems or limited public health infrastructure, and small outbreaks can escalate fast.
Let’s not forget: plague can travel. While bubonic plague is the most common and least contagious form, if the infection reaches the lungs, it becomes pneumonic—and suddenly, it spreads from person to person through respiratory droplets. That’s how localized cases can tip toward public health emergencies, particularly in remote or underserved regions.
The question isn’t just where plague is—it’s where it could go, if unnoticed or unmanaged. That’s why modern surveillance programs track rodent die-offs, monitor flea populations, and watch for signs of plague resurgence. In places like Madagascar or the Democratic Republic of the Congo, early detection can mean the difference between a handful of cases and a serious outbreak.
So yes, plague is rare—but it’s not gone. It’s patient. It’s persistent. And it’s still very much part of the global disease landscape.
The next logical question is—what does plague actually look like in the body? Let’s talk about clinical manifestations.
Clinical Manifestations — What Happens When Plague Strikes the Body?
So what does it actually feel like to get the plague?
That’s not a question most people think they’ll need to ask. And hopefully, they never will. But to understand just how dangerous Yersinia pestis really is, we need to look at what it does once it gets inside the human body. Spoiler: it doesn’t take its time.
There’s a strange paradox at play here. For a disease so historically dramatic, its early symptoms are… pretty ordinary. Fever. Headache. Fatigue. Sometimes muscle aches. Nothing that screams “medieval killer.” If anything, the initial phase could be mistaken for the flu—or even just a bad day.
But then things escalate. Fast.
Let’s start with the bubonic plague, the most iconic and most common form. This one begins when the bacteria enter through the skin—typically after a flea bite—and travel through the lymphatic system. Within a few days, lymph nodes near the site of entry swell up massively. Painfully. These swollen, tender lumps—called buboes—usually appear in the groin, armpit, or neck, depending on where the bite occurred. And here’s the thing: they’re not subtle. We’re talking golf ball-sized, black-and-blue, and exquisitely painful. This is where the plague starts to show its teeth.
But not all infections stay localized.
If Y. pestis breaks into the bloodstream, you’ve got septicemic plague. No buboes. No visual warning. Just a full-body bacterial invasion. The symptoms here are more systemic: chills, abdominal pain, bleeding under the skin, even gangrene in the fingers and toes. It’s brutal—and without treatment, almost universally fatal. The lack of a clear early sign? That’s what makes it so dangerous. It hides while it kills.
And then there’s the worst-case scenario: pneumonic plague. This form happens when the bacteria reach the lungs—either from within the body or by inhaling droplets from another infected person. Now it’s not just deadly, it’s contagious. Highly contagious. This is the form that can spark outbreaks. The symptoms hit hard: cough, chest pain, shortness of breath, and frothy or bloody sputum. Patients can go from initial cough to severe respiratory failure in under 48 hours.
You might wonder: are these forms totally separate diseases? Not exactly. They’re different expressions of the same bacterial assault, shaped by where the bug goes and how fast it spreads. One form can evolve into another, and some patients might show overlapping symptoms. It’s messy. Medicine doesn’t always fit into neat diagnostic boxes.
So how does the body respond to all this?
The immune system tries to fight back. But as we saw earlier, Y. pestis is a tactician. It neutralizes immune cells, interferes with signaling pathways, and triggers a storm of inflammation. Ironically, sometimes it’s the immune system’s own overreaction that worsens the damage—especially in the lungs.
If left untreated, mortality rates can be shocking: up to 60% for bubonic, nearly 100% for septicemic and pneumonic. With antibiotics? The numbers drop dramatically. But that’s assuming early detection and rapid response—which isn’t always guaranteed, especially in resource-limited settings.
And that leads to the next critical question: how do we actually diagnose the plague—especially before it’s too late?
Let’s head there next.
Diagnosis — How Do You Catch a Killer Before It’s Too Late?
If someone comes into a clinic with a fever and swollen lymph nodes, how do doctors know it’s plague and not just a nasty flu or some other infection? That’s a big challenge. Because early plague symptoms are often vague, doctors need a sharp eye and good tools to make the call before the disease gets out of control.
So what’s the first step? Usually, it starts with clinical suspicion. If a patient lives in or has recently traveled to a plague-endemic area, and they show the classic signs—like those swollen buboes, sudden high fever, or unexplained pneumonia—clinicians start thinking about Yersinia pestis. But outside of known hotspots, it’s easy to miss. After all, how often does anyone expect to see plague in the 21st century?
That’s why laboratory testing is crucial. But here’s where things get complicated.
One traditional method is to take samples—like blood, lymph node aspirate, or sputum—and stain them with special dyes to look for Y. pestis under the microscope. It’s fast, but not foolproof. The bacteria can be hard to spot early on, and sometimes the sample just doesn’t have enough bacteria to detect.
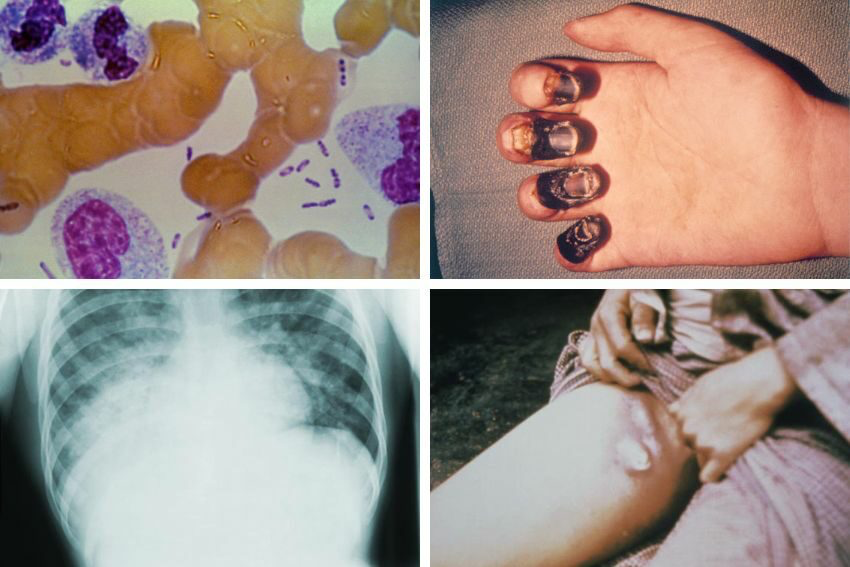
Then there’s culture—growing the bacteria in the lab from patient samples. This method is more definitive but takes time, often several days, which isn’t ideal when every hour counts. Plus, it requires a biosafety level 3 lab because Y. pestis is so dangerous, limiting its availability.
More recently, molecular techniques like PCR (polymerase chain reaction) have revolutionized diagnosis. These tests look for the bacterium’s DNA directly and can give results in hours. But not every clinic or hospital worldwide has access to these advanced tools, especially in rural or resource-poor settings where plague often strikes.
Serology—testing for antibodies against Y. pestis—can confirm infection after the fact, but it’s less useful for early diagnosis when treatment decisions must be made immediately.
So what happens if early diagnosis fails or is delayed? Sadly, that’s often when patients present in severe stages—septicemic or pneumonic plague—making treatment harder and outcomes worse.
Given all these hurdles, you might wonder: how do public health officials keep track of plague cases? The answer is through surveillance and reporting systems—networks of healthcare workers, labs, and epidemiologists who watch for signs of plague in animals and humans. They also monitor unusual rodent die-offs, which can signal an outbreak before it spills over to people.
That said, even the best surveillance isn’t perfect. Plague’s ability to mimic other diseases and its rapid progression mean diagnosis remains a high-stakes race.
Which naturally brings us to the next question: once diagnosed, how do we fight it? How do you treat a disease that can kill so quickly if you don’t act fast?
Let’s explore that.
Treatment and Management — Can Modern Medicine Beat an Ancient Killer?
When someone is diagnosed with plague, the clock starts ticking. How do doctors turn the tide against such a ruthless microbe? It’s one of those moments where science and urgency collide.
First off, you might ask: is plague still treatable? The answer—thankfully—is yes. Antibiotics are the frontline weapons here, and when started early enough, they can drastically reduce mortality. But timing is everything.
Which antibiotics work best? Historically, streptomycin has been the gold standard, but it’s not always readily available. Alternatives like gentamicin, doxycycline, and ciprofloxacin have also proven effective and are used depending on availability and patient factors. Treatment usually lasts at least 10 days, sometimes longer, to make sure every last bacterium is wiped out.
But treatment isn’t just about the antibiotics. Think about it: the body is already under siege—fever, inflammation, and sometimes organ failure. That means patients often need supportive care: intravenous fluids to counter dehydration, oxygen therapy if lungs are involved, and close monitoring for complications like septic shock.
Now, what happens if the disease has progressed too far? Septicemic and pneumonic plague cases can deteriorate rapidly, sometimes despite antibiotics. The immune system’s overreaction—cytokine storms, widespread inflammation—can be just as deadly as the bacteria itself. Managing these complications often requires intensive care, but even then, the fatality rate remains high without early intervention.
This leads to another crucial question: could Yersinia pestis become resistant to antibiotics? Resistance is a nightmare in infectious disease management. For plague, antibiotic resistance remains rare, but it’s not impossible. Scientists keep a close eye on this because if resistance emerges, it could dramatically change treatment strategies—and not for the better.
Are there any vaccines to help prevent plague? Historically, plague vaccines have existed but none are widely used today because they offer only partial protection and can have side effects. Research continues, but for now, vaccination isn’t a primary tool in most regions.
So treatment hinges on early diagnosis and rapid antibiotic therapy combined with good supportive care.
But treatment is just one piece of the puzzle. How do we stop plague before it ever reaches a human host?
That’s the realm of prevention and control.
Prevention and Control — How Do We Keep the Plague From Striking Back?
If treatment is about fighting a losing battle once plague arrives, prevention is about stopping it before it even gets a foothold. But what does prevention look like against a disease with such a complex lifecycle and animal reservoirs?
Let’s start with the obvious: can we eliminate plague completely? Given that Yersinia pestis is deeply embedded in wild rodent populations across continents, eradication isn’t realistic—at least not yet. The bacterium has its own natural cycles independent of humans. So instead, the focus is on control: minimizing human risk and quickly containing outbreaks.
One major pillar of prevention is reducing human contact with infected fleas and rodents. That sounds simple, but it’s easier said than done. Rodents are everywhere, especially in rural or poor regions where housing might be less secure against pests. Fleas hitch rides on animals and can invade homes. So public health efforts often include rodent control programs, insecticide spraying, and educating communities about avoiding contact with sick or dead animals.
Which raises a key question: how do you get people to change behaviors rooted in their daily lives and environment? Public health campaigns must be culturally sensitive and practical. Telling people to avoid their livestock or wild rodents is one thing; ensuring they have the means to do so is another.
Then there’s surveillance—the cornerstone of modern plague control. This means constant monitoring of rodent populations, flea indices, and human cases. In places like Madagascar or parts of the US Southwest, early detection systems watch for sudden spikes in rodent deaths, which often precede human cases. When these alarms go off, health officials can deploy targeted interventions fast.
What about quarantine and isolation? In the case of pneumonic plague, which can spread person-to-person through respiratory droplets, isolating patients and using protective equipment becomes vital. But such measures require infrastructure, trained personnel, and public compliance—resources that aren’t always available where plague strikes hardest.
Does climate play a role? Absolutely. Changes in temperature, rainfall, and habitat can influence rodent and flea populations, potentially increasing plague risk. This intersection of ecology and epidemiology means plague outbreaks can be somewhat seasonal or tied to environmental shifts, adding another layer to prevention efforts.
So prevention isn’t just a medical issue—it’s ecological, social, and logistical. It’s about building systems that detect and respond quickly, educating at-risk populations, and managing the environment as much as the bacteria itself.
With so many moving parts, it’s no wonder plague has persisted for centuries despite our best efforts.
Which brings us to the cutting edge: how are recent advances changing the game?
Recent Developments (2025–2026) — What’s New in the World of Plague?
You might think that after centuries, plague would be a solved mystery. But far from it. The story of Yersinia pestis is still unfolding, and recent years have brought some eye-opening developments that remind us this ancient foe is far from defeated.
First off, let’s talk outbreaks. While global plague cases remain relatively low, there have been notable flare-ups recently—in Madagascar, the Democratic Republic of the Congo, and even unexpected isolated cases in the United States. These remind us that plague doesn’t only belong in dusty history books. It can and does flare up, sometimes explosively.
What’s driving these recent outbreaks? The answer is complex. Climate shifts altering rodent habitats, increased human encroachment into wilderness areas, and challenges in healthcare access all play a role. Plus, socio-political instability in some regions complicates public health responses. In other words, plague thrives in the cracks where surveillance, infrastructure, and resources falter.
On the technology front, diagnostics have seen exciting improvements. Rapid molecular tests now offer faster, more reliable detection in the field. Imagine a handheld device that can identify Yersinia pestis DNA in a matter of hours, not days. This is transforming outbreak management, allowing for quicker containment and treatment.
Treatment, too, is evolving. Researchers are exploring new antibiotic regimens, combination therapies, and adjunct treatments aimed at modulating the immune response. There’s even work on developing better vaccines—ones that could offer stronger and longer-lasting immunity with fewer side effects.
But here’s a question that keeps scientists awake at night: could Y. pestis change enough to become more resistant or more transmissible? So far, resistant strains are rare, but the possibility remains. Continuous genomic surveillance is crucial to catch these changes early.
Another intriguing frontier is understanding how plague interacts with the microbiome—the vast community of microbes living in and on our bodies. Could some of these microbes influence susceptibility or severity? It’s early days, but the answer could open new pathways for prevention or treatment.
So despite centuries of research, plague remains a moving target. New tools and knowledge are helping us catch up, but Yersinia pestis still keeps a few tricks up its sleeve.
As we wrap up, what’s the takeaway? Vigilance is non-negotiable. Preparedness means investing in surveillance, healthcare infrastructure, and public education—even for diseases that seem ancient or rare. Because when it comes to plague, history is a warning and a guide.
Next, let’s pull it all together.
FAQ: Key Questions About Plague (Yersinia pestis)
What is Yersinia pestis and why is it still important today?
Yersinia pestis is the bacterium responsible for plague, an ancient disease that caused massive pandemics like the Black Death. It remains important because it still exists in natural reservoirs and can cause outbreaks, especially in certain regions.
How does Yersinia pestis infect humans and cause disease?
It enters the body typically through flea bites, then evades and disables the immune system, spreading through lymph nodes, blood, or lungs, causing bubonic, septicemic, or pneumonic plague.
Where is plague still found, and who is at risk?
Plague persists mainly in parts of Africa, Asia, and the Americas, often in rural areas near wild rodents. People with close contact to infected animals or fleas—like farmers, hunters, and park workers—are most at risk.
What are the symptoms of plague?
Symptoms vary by form but include fever, swollen and painful lymph nodes (buboes), severe systemic illness, and in pneumonic plague, respiratory distress and coughing up blood.
How is plague diagnosed?
Diagnosis relies on clinical suspicion, microscopic examination, bacterial culture, and increasingly on rapid molecular tests like PCR. Early diagnosis is challenging but critical.
Can plague be treated?
Yes. Antibiotics like streptomycin, gentamicin, and doxycycline are effective if started early, along with supportive care. Delay in treatment greatly increases mortality risk.
How can plague be prevented?
Preventive measures include controlling rodent and flea populations, reducing human contact with wild reservoirs, using insect repellents, wearing protective clothing, and monitoring animal populations for signs of plague.
Are there vaccines against plague?
Vaccines exist but are not widely used due to limited protection and side effects. Research continues to develop better vaccines.
Has plague changed recently?
Recent outbreaks remind us plague is still active. Advances in rapid diagnostics and treatment are promising, but ongoing surveillance is necessary to detect changes like antibiotic resistance.
Why is plague still a public health concern?
Because it persists in wildlife reservoirs, can cause severe outbreaks if not quickly contained, and requires vigilance in monitoring and response—even in an era of advanced medicine.
Final Thoughts
Plague may feel like a disease of the distant past, but its story is far from over. Yersinia pestis is a remarkable example of how ancient pathogens can persist, adapt, and remind us of the delicate balance between humans, animals, and the environment. Its survival in hidden reservoirs, combined with the speed and severity of disease it can cause, means that vigilance is crucial. Early recognition, rapid diagnosis, effective treatment, and thoughtful prevention strategies all form the pillars that keep this ancient killer at bay.
While modern medicine has given us powerful tools, Y. pestis teaches us humility: that the microscopic world still holds surprises, and that staying ahead means never letting our guard down. It’s a message that resonates across outbreaks—whether bacterial or viral. Our recent feature on Ebola examines similar challenges: remote emergence, rapid escalation, and the vital need for infrastructure and trust during crisis response.
Understanding plague is more than a historical curiosity—it’s a vital lesson in preparedness, ecological awareness, and the ongoing challenges of infectious diseases in a connected world. While modern medicine has given us powerful tools, Yersinia pestis teaches us humility: that the microscopic world still holds surprises, and that staying ahead means never letting our guard down.