PI-RADS 4 Prostate Cancer: Survival Rates and Clinical Insights
- Understanding PI-RADS and Its Role in Prostate Cancer Diagnosis
- Clinical Significance of a PI-RADS 4 Score
- Survival Rates Associated with PI-RADS 4 Lesions
- Diagnostic Pathways Following a PI-RADS 4 Finding
- Treatment Options After Confirming a Cancer Diagnosis
- Active Surveillance vs. Aggressive Management
- Prognostic Factors Influencing Survival
- Five-Year Survival Estimates by Risk Category
- Role of Androgen Deprivation Therapy (ADT) in PI-RADS 4 Cases
- Managing Side Effects and Quality of Life
- Post-Treatment Surveillance and Monitoring
- Biochemical Recurrence and Salvage Treatment Strategies
- International Perspectives and Treatment Variability
- Psychological and Emotional Impact of a PI-RADS 4 Finding
- Emerging Research and Evolving Standards
- Conclusion: Proactive Care Begins with Interpretation
- FAQ
Understanding PI-RADS and Its Role in Prostate Cancer Diagnosis
PI-RADS, short for Prostate Imaging Reporting and Data System, is a globally accepted classification system used to interpret prostate MRI findings. Developed to improve diagnostic consistency across radiologists and urologists, PI-RADS assigns a score from 1 to 5 to any suspicious lesion based on how likely it is to represent clinically significant prostate cancer. A score of 4 reflects a high suspicion, meaning there is a strong probability of detecting cancer that requires treatment rather than monitoring.
The system relies on multiparametric MRI, which evaluates tissue through several advanced imaging sequences. This technique allows radiologists to differentiate between benign prostatic changes and potentially aggressive disease. With increasing use, PI-RADS scoring has become instrumental in minimizing unnecessary biopsies while ensuring high-risk lesions are not missed.
Interestingly, just as radiological systems like PI-RADS help predict cellular risk patterns in oncology, discussions about subtle biological stimulation — such as in therapies covered in the article Can Microcurrent Cause Cancer — demonstrate how modern medicine continues to evolve in risk stratification and early intervention frameworks.
Clinical Significance of a PI-RADS 4 Score
When a radiologist assigns a PI-RADS 4 score, it indicates that the lesion in question has imaging characteristics strongly suggestive of cancer. Statistically, around 60–80% of these lesions are confirmed as prostate cancer upon biopsy, often with moderate to high Gleason grades. The concern is not only the presence of cancer but whether it is likely to grow quickly or spread beyond the prostate.
From a clinical standpoint, a PI-RADS 4 lesion typically triggers further diagnostic workup without delay. Urologists often prioritize such findings for targeted biopsy, followed by genetic profiling or additional imaging if warranted. These steps help determine whether the cancer is localized, locally advanced, or already metastatic.
It’s worth emphasizing that not all PI-RADS 4 lesions lead to a diagnosis of lethal disease. Some may represent low-volume, indolent cancers that are still eligible for active surveillance. However, due to the statistical likelihood of significant pathology, the default approach is more aggressive evaluation and early intervention, aimed at preserving survival odds and treatment options.
Survival Rates Associated with PI-RADS 4 Lesions
Although PI-RADS 4 is not a staging label, it often corresponds with intermediate to high-risk categories once pathology results are available. When cancer is confined to the prostate and identified early, survival rates remain exceptionally high — five-year survival typically exceeds 95%, especially with immediate treatment. However, the deeper concern with PI-RADS 4 lesions lies in the potential for higher Gleason scores, extracapsular extension, or involvement of the seminal vesicles.
Survival outcomes vary significantly based on how quickly the lesion is biopsied and treated. For instance, patients with PI-RADS 4 findings who delay biopsy or opt for prolonged monitoring have a higher risk of discovering advanced-stage disease upon eventual treatment. This makes the score both a diagnostic marker and a clinical urgency signal.
Just as in other solid tumors like pancreatic malignancies, where early-stage detection plays a decisive role in prognosis, timely action following a PI-RADS 4 designation can be the dividing line between curative and palliative care. The survival discussion must be individualized, incorporating PSA levels, tumor grade, and patient age — but the importance of the imaging score as a predictive flag cannot be overstated.
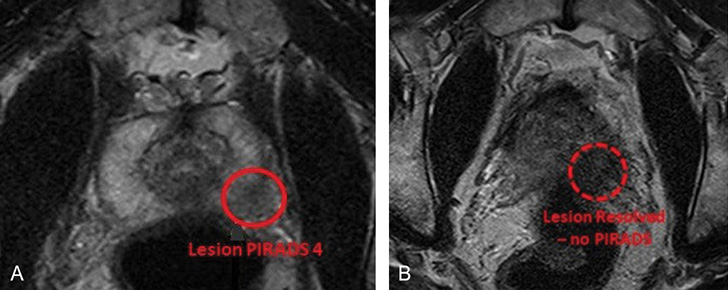
Diagnostic Pathways Following a PI-RADS 4 Finding
The diagnostic cascade begins immediately after a PI-RADS 4 lesion is identified on MRI. A targeted biopsy is usually performed, guided either by cognitive fusion (where the MRI image is mentally mapped during ultrasound) or by MRI-ultrasound fusion software. These methods improve sampling accuracy and reduce the risk of missing aggressive areas within the gland.
Once tissue is obtained, pathologists assess it for malignancy, determine the Gleason score, and evaluate margins or invasion patterns. Concurrent PSA levels and digital rectal exams further contextualize the lesion’s threat. Some patients may also undergo bone scans or pelvic CTs to rule out regional or distant metastases, particularly if PSA is elevated.
It’s worth noting that the evolution of imaging-guided diagnostics — from PI-RADS to fusion biopsies — mirrors advances in other cancer fields. For example, early imaging evaluation is just as critical in conditions such as pancreatic or ovarian cancer, where vague symptoms require precise anatomical targeting for meaningful intervention, as covered in First Symptoms of Ovarian Cancer.
Treatment Options After Confirming a Cancer Diagnosis
Once a PI-RADS 4 lesion has been biopsied and confirmed to be malignant, treatment decisions are based on the Gleason score, PSA level, cancer stage, patient age, and comorbidities. For localized prostate cancer with favorable risk features, options include active surveillance, radical prostatectomy, or external beam radiation therapy. For intermediate- to high-risk disease — which is more likely with a PI-RADS 4 lesion — combination therapy is often considered.
Radical prostatectomy is a standard surgical option, especially for men under 70 with a long life expectancy. Advances in minimally invasive approaches, such as robotic-assisted laparoscopic surgery, have reduced recovery times and surgical complications. Alternatively, high-dose radiation therapy, often combined with short- or long-term androgen deprivation therapy (ADT), offers curative potential without surgery. The decision between surgery and radiation is patient-specific and should be made in collaboration with a multidisciplinary team.
Because prostate tumors vary biologically, many centers now recommend genomic testing to identify aggressiveness at the molecular level. This guides not only treatment intensity but also the need for additional imaging or adjunct therapies.
Active Surveillance vs. Aggressive Management
For some men, especially those with lower-volume or favorable-risk prostate cancer, active surveillance remains a valid strategy — even after a PI-RADS 4 designation. Surveillance involves regular PSA testing, MRI imaging, and repeat biopsies to monitor for progression. The goal is to delay or avoid treatment and its side effects while maintaining safety.
However, this approach is only appropriate when the biopsy confirms low-grade cancer (e.g., Gleason 6) despite the suspicious imaging score. If the pathology shows Gleason 7 or higher, or if PSA continues to rise, most clinicians recommend definitive treatment. The transition from surveillance to intervention is based on disease progression, patient anxiety, and evolving clinical markers.
There is a subtle but essential clinical parallel here: like in pancreatic cancer, where observation is rarely feasible due to the aggressive natural history of the disease, certain prostate cancer profiles demand immediate action. In both contexts, imaging findings — whether it’s a PI-RADS 4 lesion or a pancreatic mass — carry weight not just diagnostically, but therapeutically as well, as discussed in Pancreatic Cancer.
Prognostic Factors Influencing Survival
Several critical variables affect long-term survival after a PI-RADS 4 diagnosis. First and foremost is the Gleason score, which remains the strongest pathological predictor of recurrence and mortality. A score of 6 suggests indolent disease, while a score of 8 or higher is associated with a high risk of progression and metastasis. The PSA level at diagnosis, particularly if it exceeds 20 ng/mL, also predicts more aggressive disease.
Tumor volume and location play roles too. Lesions located in the anterior prostate or those that involve the seminal vesicles carry worse prognoses. Moreover, genetic mutations such as BRCA2 or ATM — found through molecular profiling — are increasingly recognized as indicators of aggressive biology and lower survival outcomes.
Lastly, patient age, overall health, and access to treatment centers capable of delivering complex care contribute to survival differences across populations. Early-stage disease treated promptly generally leads to excellent long-term control, while delays in diagnosis or limited access to therapy can dramatically alter the survival curve.
Five-Year Survival Estimates by Risk Category
| Risk Group | PSA (ng/mL) | Gleason Score | Tumor Stage | Estimated 5-Year Survival |
| Low Risk | <10 | ≤6 | T1–T2a | >98% |
| Favorable Intermediate | 10–20 | 3+4=7 | T2b–T2c | 95–98% |
| Unfavorable Intermediate | 10–20 | 4+3=7 | T2c–T3a | 85–92% |
| High Risk | >20 | 8–10 | ≥T3b | 65–80% |
| Very High Risk | Any | 9–10 | T4, N1 | 50–65% |
These figures are estimates based on population-level data and assume access to contemporary treatment. They are most relevant to men diagnosed early and treated appropriately. The presence of a PI-RADS 4 lesion often aligns with intermediate or high-risk categories, especially if coupled with adverse pathology, thus highlighting the need for a rapid, individualized response.
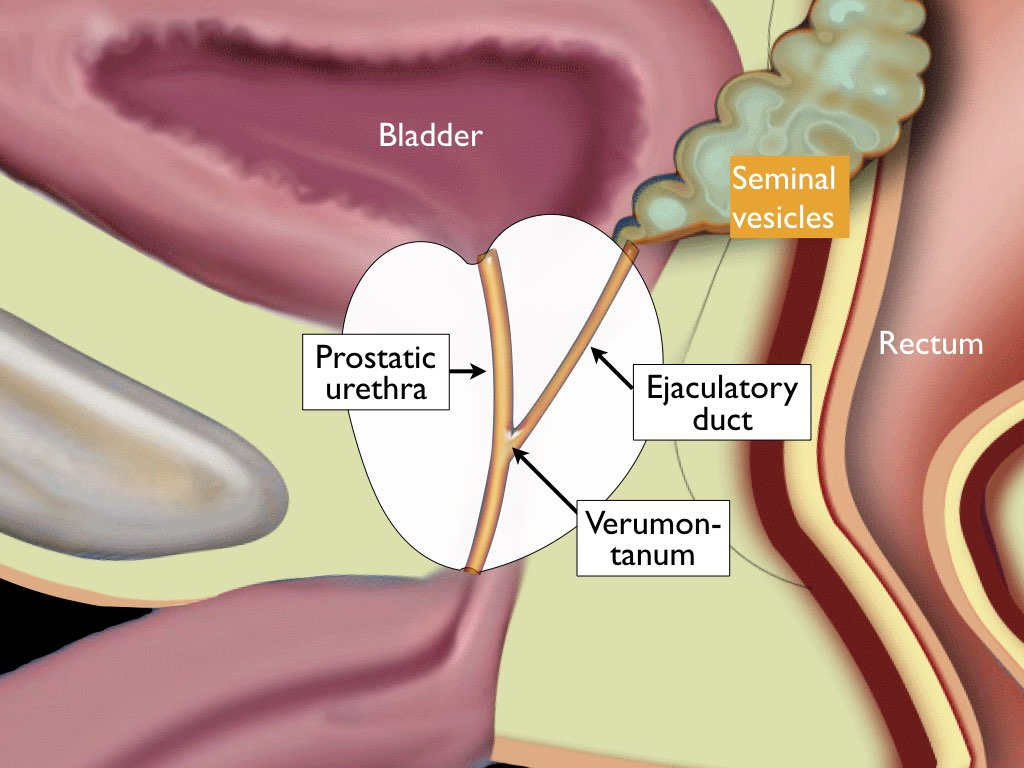
Role of Androgen Deprivation Therapy (ADT) in PI-RADS 4 Cases
Androgen deprivation therapy remains a foundational treatment for intermediate and high-risk prostate cancer, particularly when combined with radiation therapy. Since prostate cancer cells rely on testosterone to grow, lowering androgen levels slows disease progression. In the context of a PI-RADS 4 lesion with confirmed high-grade cancer, ADT may be introduced prior to radiation or as an adjunct in metastatic scenarios.
ADT is typically administered through GnRH agonists or antagonists, which suppress testosterone production. In some cases, surgical orchiectomy may be considered, though this is less common in modern practice. Duration of hormonal therapy is tailored based on risk level — six months for intermediate risk and up to two to three years for high-risk disease.
While effective, ADT is associated with significant side effects including fatigue, hot flashes, loss of libido, weight gain, and bone density reduction. Regular monitoring for metabolic complications is necessary, and patients may benefit from concurrent lifestyle interventions or pharmacologic support, such as bisphosphonates or antidepressants, depending on symptom burden.
Managing Side Effects and Quality of Life
Treatment-related side effects — whether from surgery, radiation, or hormonal therapy — can significantly affect a patient’s quality of life. One of the most commonly reported issues is urinary incontinence, especially after radical prostatectomy. Though many men regain control within a few months, others require pelvic floor rehabilitation or even surgical correction. Erectile dysfunction is another frequent consequence, with nerve-sparing techniques reducing but not eliminating the risk.
Radiation therapy may also lead to bladder or rectal irritation, resulting in urinary frequency, urgency, or mild bleeding. Bowel changes, such as diarrhea or tenesmus, can persist for weeks post-treatment. Hormonal therapy, meanwhile, introduces systemic effects that may compound fatigue or emotional challenges.
Addressing these complications requires a team approach. Urologists, radiation oncologists, physical therapists, mental health counselors, and sexual health specialists all play a role. Early intervention and honest communication are vital in restoring function and emotional well-being. Long-term survivorship often hinges not only on eradicating disease but also on mitigating the chronic side effects of its treatment.
Post-Treatment Surveillance and Monitoring
After primary therapy, patients enter a surveillance phase designed to detect recurrence early. This includes regular PSA testing every 3–6 months for the first few years, tapering in frequency thereafter. Any detectable rise in PSA following surgery (known as biochemical recurrence) warrants further investigation to determine if salvage radiation or systemic therapy is needed.
Imaging may also be repeated depending on the risk of recurrence, particularly in those with high initial tumor burden or adverse pathology. In some cases, advanced PET imaging techniques, such as PSMA-PET, are used to identify microscopic metastases earlier than conventional scans.
Psychological monitoring is also crucial in survivorship. Anxiety surrounding follow-up appointments, fear of recurrence, and the long-term implications of cancer diagnosis often persist long after treatment concludes. Offering structured survivorship plans that address emotional, medical, and logistical challenges can significantly improve patient experience during this stage.
Biochemical Recurrence and Salvage Treatment Strategies
Biochemical recurrence — defined as a rising PSA after surgery or radiation — does not always equate to clinical relapse, but it signals a need for action. The timing and pace of PSA increase guide next steps. If recurrence is local and confined to the prostate bed, salvage radiation may be effective. If recurrence is systemic, hormone therapy or novel agents like androgen receptor blockers may be introduced.
The decision to initiate salvage therapy is individualized, based on PSA doubling time, Gleason score, patient age, and time since initial treatment. Not all recurrences require immediate intervention; in some cases, observation remains a valid option. However, delaying treatment without careful monitoring can lead to missed opportunities for secondary control.
Advanced molecular imaging and liquid biopsy technologies are increasingly used to detect recurrence earlier and identify those most likely to benefit from salvage therapy. As research progresses, these tools are expected to improve both the timing and personalization of treatment for patients experiencing PSA relapse.
International Perspectives and Treatment Variability
Prostate cancer management strategies, including the interpretation and response to PI-RADS 4 lesions, can differ significantly between countries. In the United States and much of Western Europe, early MRI-guided biopsy and risk-adapted therapy have become standard. Multidisciplinary tumor boards regularly guide treatment decisions, ensuring alignment between radiological findings and oncologic planning.
However, in many parts of the world, access to multiparametric MRI or fusion biopsy remains limited. In such regions, elevated PSA or digital rectal exam findings may still prompt systematic biopsy without the benefit of precise lesion localization. As a result, overdiagnosis and underdiagnosis continue to be problems, particularly in low-resource settings.
Comparing these approaches underscores the role of health systems in shaping cancer outcomes. Just as early imaging access influences survival in prostate cancer, similar disparities exist in conditions like ovarian and pancreatic cancer, where early identification is often the dividing line between curability and chronicity. Bridging global gaps in imaging and pathology access remains a key priority for international cancer policy.
Psychological and Emotional Impact of a PI-RADS 4 Finding
Receiving a PI-RADS 4 score can provoke intense anxiety, even before a cancer diagnosis is confirmed. The knowledge that the lesion is “likely significant” — and the implications it may carry — often leads to anticipatory distress, sleep disturbances, and difficulty concentrating. For many men, the psychological burden begins with the MRI report and persists throughout biopsy, staging, and treatment.
Younger patients may experience additional concerns around fertility, sexual identity, or career disruptions, while older individuals often grapple with fears of mortality or treatment complications. These emotional responses are valid and deserve structured attention.
Incorporating mental health screening into the prostate cancer care pathway — ideally before biopsy — helps address this burden proactively. Support groups, oncology-focused counseling, and peer mentorship can normalize the experience and reduce isolation. Just as patients facing other serious diagnoses, like pancreatic cancer or advanced breast cancer, benefit from early psychological support, men with PI-RADS 4 lesions deserve the same integrated care model.
Emerging Research and Evolving Standards
Prostate cancer research is entering a new era, with studies now focusing on artificial intelligence-driven image analysis, PSMA-targeted therapies, and next-generation risk calculators that combine MRI, genomics, and biomarkers. These advances aim to refine decision-making, reduce unnecessary biopsies, and improve detection of truly aggressive cancers.
Clinical trials increasingly stratify patients based on imaging scores like PI-RADS, integrating radiological risk into trial design. New hormonal agents and radiopharmaceuticals, such as lutetium-177 PSMA therapy, show promise in treating advanced cases with less toxicity than traditional chemotherapy. At the same time, machine learning algorithms are being trained to identify malignancy patterns within MRI scans, potentially offering even earlier warnings than radiologist-assigned scores.
For patients with PI-RADS 4 lesions, these innovations may offer new options — both in terms of conservative management and cutting-edge interventions. Participation in clinical research, even at the point of biopsy, could soon become a standard recommendation, as it is in other evolving oncologic fields.
Conclusion: Proactive Care Begins with Interpretation
A PI-RADS 4 lesion is not a diagnosis, but it is a loud signal — one that must not be ignored. It represents a high likelihood of clinically significant prostate cancer, and with it comes the opportunity to act early, personalize care, and improve survival outcomes. What follows after this score — biopsy, treatment, monitoring — can determine years of quality life or months of preventable regret.
From the moment a PI-RADS 4 score appears on a radiology report, the patient enters a critical decision window. Prompt investigation, honest dialogue with care teams, and shared decision-making are the strongest tools available. And with the rapid advances in imaging, pathology, and therapeutics, the outlook for men with this finding is improving steadily.
While the challenges are real — biologically, psychologically, and systemically — the path forward is guided by clarity, evidence, and compassion. For men facing a PI-RADS 4 result, the journey begins not in fear, but in focused readiness.
FAQ
What does a PI-RADS 4 score actually mean?
A PI-RADS 4 score suggests a high likelihood — approximately 60–80% — of clinically significant prostate cancer. It means that the lesion seen on MRI has imaging characteristics highly suspicious for cancer, and a biopsy is strongly recommended to confirm or rule out the diagnosis.
Does a PI-RADS 4 lesion always mean I have cancer?
No, although a PI-RADS 4 lesion indicates a strong suspicion, not every such lesion is cancerous. Some turn out to be benign after biopsy, such as inflammation or hyperplasia. However, due to the high probability of cancer, follow-up with biopsy is essential.
How soon should I get a biopsy after receiving a PI-RADS 4 result?
Ideally, a biopsy should be scheduled promptly, usually within a few weeks. Delaying the biopsy may allow potentially aggressive cancer to grow or spread. Early biopsy ensures timely diagnosis and faster access to treatment options if needed.
What is the survival rate if cancer is confirmed after a PI-RADS 4 score?
If cancer is localized and treated early, five-year survival rates exceed 95%. However, if the tumor is high-grade or has already extended beyond the prostate, survival drops significantly. The PI-RADS score itself is not a prognosis but a marker for likelihood of significant disease.
Can I still choose active surveillance with a PI-RADS 4 score?
Yes, but only if the biopsy shows low-risk, low-grade cancer (e.g., Gleason 6) and your PSA is stable. In these cases, active surveillance may be appropriate. However, most PI-RADS 4 lesions are associated with more aggressive disease requiring treatment.
What kind of biopsy is best for PI-RADS 4 lesions?
Targeted biopsy using MRI-ultrasound fusion offers the highest accuracy, especially when guided by the lesion identified on MRI. This approach increases the likelihood of sampling the correct area and reduces false negatives compared to random sampling.
How is a PI-RADS 4 lesion different from PI-RADS 3 or 5?
PI-RADS 3 indicates intermediate suspicion (15–25% chance of significant cancer), often leading to watchful waiting or repeat imaging. PI-RADS 5 implies very high suspicion, with a >90% likelihood of aggressive cancer. PI-RADS 4 sits between these two in terms of risk.
Will I need treatment immediately after a positive biopsy?
Not necessarily. Treatment urgency depends on tumor grade, stage, PSA level, and your overall health. Some patients may opt for immediate surgery or radiation, while others may pursue short-term observation while undergoing additional evaluations.
Can I get a second opinion on my MRI or biopsy?
Yes. Second opinions from a radiologist or urologist are common and encouraged, especially for complex or borderline cases. A second review of MRI images can confirm the accuracy of the PI-RADS score and clarify the best next steps.
Does a PI-RADS 4 lesion always lead to surgery?
No. Surgery is one treatment option, but others include radiation therapy, hormone therapy, or focal treatments. The choice depends on cancer aggressiveness, patient preference, age, and side effect profiles.
How accurate is the PI-RADS system overall?
PI-RADS is highly effective, especially when interpreted by experienced radiologists. While it doesn’t guarantee a cancer diagnosis, it significantly improves the ability to identify patients who need further testing and reduces unnecessary biopsies in low-risk cases.
Is PI-RADS used outside the U.S.?
Yes. PI-RADS is an internationally accepted system used across Europe, Asia, Australia, and other regions. It ensures standardized communication between radiologists and clinicians globally, improving consistency in diagnosis and care.
Are there risks in waiting too long to act on a PI-RADS 4 lesion?
Yes. Delay in biopsy or treatment can allow significant cancer to progress, potentially moving from curable to incurable. Prompt evaluation is key, especially for lesions with high PI-RADS scores.
How does this relate to genetic cancer risk?
A PI-RADS 4 lesion may warrant genetic testing, particularly if you have a family history of prostate, breast, or pancreatic cancer. Mutations like BRCA2 or ATM increase the risk of aggressive prostate cancer and may influence treatment choices.
Can lifestyle changes reduce my risk if PI-RADS 4 leads to a negative biopsy?
Yes. Although you can’t eliminate all risk, adopting a healthy diet, regular physical activity, avoiding smoking, and managing stress can support prostate health. Continued monitoring with PSA and repeat imaging remains essential in high-risk individuals.









