Oropouche Fever: An Emerging Arboviral Threat
- Part One – Introduction: Oropouche Fever – An Emerging Arboviral Threat
- Part Two – Virology and Pathogenesis: What’s Oropouche Virus Really Made Of?
- Part Three – Epidemiology: Where Oropouche Travels and Who It Touches
- Part Four – Clinical Manifestations: What It Feels Like When Oropouche Hits
- Part Five – Diagnosis: Knowing It’s Oropouche (When It Looks Like Everything Else)
- Part Six – Treatment and Management: When There’s No Silver Bullet
- Part Seven – Prevention and Control: Stopping Oropouche Before It Starts
- Part Eight – Recent Developments (2025–2026): What’s New in the Oropouche Story?
- Oropouche Fever: Frequently Asked Questions (FAQ)
- Final Thought
Introduction: Oropouche Fever – An Emerging Arboviral Threat
You probably haven’t thought much about Oropouche virus. Most people haven’t. It’s not a household name like Zika, dengue, or chikungunya — not yet, anyway. But in the world of emerging infectious diseases, it’s quietly gaining attention, and for good reason. This isn’t a new virus, but it’s becoming newly relevant.
Oropouche virus, or OROV for short, sits within the Orthobunyavirus genus, a subset of the Peribunyaviridae family. That might sound like taxonomic trivia, but its place in that group says something important — OROV, like its viral cousins, is transmitted by tiny arthropods. In this case, midges and mosquitoes are the main players. The virus was first isolated in 1955 in Trinidad, pulled from the blood of a sick forest worker and a mosquito. That was the first documented encounter between OROV and modern science, but it wasn’t the beginning of the story — just the first time we noticed.
Since that quiet debut, the virus has made repeated appearances, particularly in Brazil, where it’s caused outbreaks involving tens of thousands of people. It tends to flare up suddenly, spread fast, and then retreat, leaving behind communities rattled by high fevers, headaches, rashes — and a lingering question: what just hit us?
For decades, Oropouche was mostly viewed as a regional curiosity. A virus of the Amazon, of the periphery. Something that showed up in journals but didn’t prompt headlines. But over the last few years, that’s been changing. The world is warming. Forests are falling. People and vectors are moving in new patterns, often toward each other. And in that shifting landscape, OROV is finding new opportunities.
It’s not just about climate, though. Advances in surveillance have improved our ability to detect these outbreaks, and as we pay closer attention, we’re realizing the virus may be far more widespread than we thought. The true number of Oropouche infections is likely underreported, hidden beneath the clinical similarity it shares with dengue and other tropical fevers. And unlike some arboviruses that stay tucked away in specific habitats or host reservoirs, OROV is happy to jump between sylvatic (jungle) and urban cycles — which makes it a much bigger challenge to control.
So here we are in 2025, talking about a virus that’s been quietly brewing in the shadows of the Amazon for over half a century. Why now? Because Oropouche isn’t just a footnote anymore. It’s an emerging player in the landscape of arboviral threats — one that doesn’t yet have a vaccine, doesn’t respond to antivirals, and still surprises us with how little we actually know about its reach.
As we move forward, understanding OROV’s biology, how it spreads, and how it makes people sick is more than academic curiosity — it’s a necessity. And that’s where we go next. Let’s open the virus up under the microscope and see what makes it tick.
Virology and Pathogenesis: What’s Oropouche Virus Really Made Of?
Let’s talk about what Oropouche virus actually is. Not just that it causes fever or belongs to some complicated-sounding genus — but what makes it tick, on a molecular level. Because once you understand the architecture of this virus, you begin to see why it spreads the way it does and why it’s so tricky to pin down.
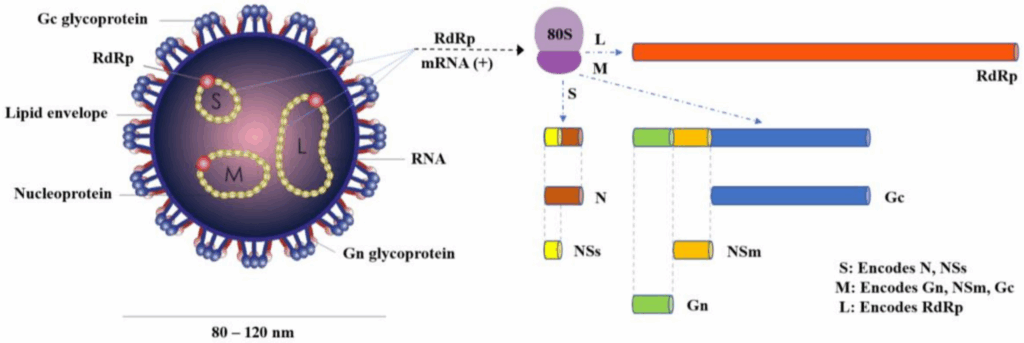
So imagine this: Oropouche virus is a tiny, spherical particle, about 100 nanometers across — so small it would take ten thousand of them lined up to match the width of a human hair. But inside this microscopic globe is a surprisingly sophisticated design. Like other orthobunyaviruses, it carries a tripartite, negative-sense RNA genome — meaning its genetic code is split into three segments, cleverly labeled S, M, and L.
Each of these segments has a job. The S segment encodes the nucleocapsid protein, which forms the inner shell of the virus and helps protect its genetic material. The M segment codes for the glycoproteins — the bits that stud the outside of the virus, like spikes on a medieval mace — and these are what help the virus latch onto and enter host cells. And the L segment? That one encodes the viral RNA polymerase, essentially the copy machine that lets the virus replicate once it’s inside.
That all sounds very textbook, but here’s the twist: this segmented setup isn’t just for efficiency — it’s also a feature that allows reassortment. In plain terms, if two different orthobunyaviruses infect the same host, they can mix and match genetic pieces. That’s a big deal. It means Oropouche virus has the genetic potential to evolve quickly, potentially swapping genes with related viruses to create new variants. That’s part of what makes it an emerging threat — it’s not locked into one form.
Once it enters the body — usually via the bite of an infected Culicoides paraensis midge or certain mosquito species — OROV doesn’t waste time. It targets cells of the immune system and endothelial tissues, and starts replicating. Within a few days, it can be circulating widely in the blood, triggering the host’s immune response — that’s when the symptoms hit.
But here’s where it gets interesting. For most people, Oropouche Fever is self-limiting. It causes a high fever, muscle pain, headaches, and sometimes a skin rash — classic arboviral fare. But in some cases, the virus crosses the blood-brain barrier and causes meningitis or meningoencephalitis. It doesn’t happen often, but when it does, it transforms the picture from a mild fever into a potential neurological emergency.
So, what determines whether someone gets just a few days of aches and chills or something much more serious? That’s still an open question. There’s evidence suggesting host immune response plays a role, possibly even genetic factors. There’s also the simple truth that viruses don’t behave the same way in every body. Some immune systems fight back effectively, while others create collateral damage — inflammation that ends up hurting the host more than the virus itself.
The immune response to OROV is, in fact, a bit of a double-edged sword. Early interferon response helps contain the virus, but if it’s delayed or dysregulated, it might contribute to the worsening of symptoms. And because the virus can persist in certain tissues, it may linger beyond the obvious symptoms — something that hasn’t been studied deeply yet, but needs attention.
What makes Oropouche pathogenesis so compelling — and unsettling — is this blend of familiarity and unpredictability. It behaves like other arboviruses, until it doesn’t. It causes a mild illness, until it doesn’t. It stays in the blood, until it crosses into the brain.
And in a world where vector habitats are shifting and viral spillovers are no longer rare events, a virus like this — compact, adaptable, capable of both subtle spread and severe outcomes — demands a deeper look. So let’s keep that momentum going. Next, we zoom out from the microscope and look at the bigger picture: where this virus is traveling, who it’s affecting, and how it’s getting around. Epidemiology is next.
Epidemiology: Where Oropouche Travels and Who It Touches
So, where exactly is Oropouche virus causing trouble? You might expect it to be confined to some dense, remote part of the Amazon, a virus tucked away in the undergrowth — but that’s not really the story anymore. In fact, what’s striking about Oropouche isn’t just where it started — it’s where it’s capable of going.
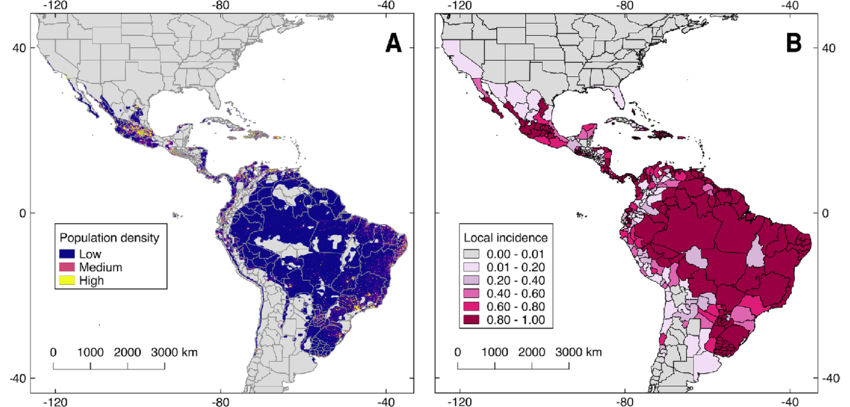
Historically, we’ve seen most outbreaks in northern and central South America, especially in Brazil, Peru, and parts of Panama and Trinidad. Some of these outbreaks have affected tens of thousands of people. Brazil, in particular, has seen repeat waves of infection — with some cities reporting hundreds of cases in just a matter of weeks. That might not sound like much on a global scale, but for a single municipality, it’s enough to overwhelm local clinics and spark alarm among health officials.
Interestingly, the dual existence of Oropouche in sylvatic and urban cycles closely echoes what we’ve observed in Plague (Yersinia pestis). Though caused by a bacterium rather than a virus, both pathogens navigate complex zoonotic pathways that make containment uniquely challenging when human development overlaps natural reservoirs.
But here’s the unsettling part: the real number of infections is probably much higher than the official data tells us. Oropouche fever often masquerades as something else — dengue, chikungunya, even the flu. Many patients with mild symptoms never get tested, and even if they do, testing for OROV isn’t always available or prioritized. So it slips through the cracks. What we’re seeing may only be the visible tip of a much deeper, more widespread phenomenon.
And it’s not just the where — it’s the who. Oropouche doesn’t target a specific demographic. It affects children and adults alike. Urban dwellers and rural villagers. What unites many of those infected isn’t their age or health status — it’s geography. They live in areas where the virus can be transmitted. And that, more than anything else, depends on the vectors.
The main vector — the one most often implicated in outbreaks — is the Culicoides paraensis, a tiny biting midge. Small enough to fit through standard mosquito nets, it’s an efficient little spreader. These midges are abundant in urban and peri-urban environments, which helps explain why OROV isn’t just a “jungle virus.” It can cause urban outbreaks, spreading quickly through densely populated areas. Certain mosquitoes, like Culex species, might also be involved in transmission, but their role is less clearly defined.
And then there’s the animal reservoir. Sloths, primates, and birds are all suspected to play a role in the sylvatic cycle — the virus’s natural circulation in forest environments. This is important, because it means that even if we control urban transmission, the virus isn’t going away. It’s still out there, in the wild, ready to be reintroduced. The boundary between forest and city has never been more porous — roads, logging, farming, migration — it all creates opportunities for spillover.
Climate is another piece of the puzzle. Rising temperatures and shifting rainfall patterns are extending the range and lifespan of insect vectors. Places that weren’t previously hospitable to these midges or mosquitoes might become welcoming. That means regions further north — perhaps even parts of Central America or the Caribbean — could start seeing local transmission under the right conditions.
And what about travel? In our globally connected world, a single infected traveler can hop on a plane in Belém and land in Miami the same day. That doesn’t mean Oropouche will immediately become a problem in North America — but it does mean the door is open. We’ve already seen it with Zika. Arboviruses move with people as much as with insects.
So here’s where we’re at: OROV is already more widespread than many realize. It’s under-diagnosed, under-reported, and underestimated. It thrives in both forest and city. It doesn’t stay put. And its ecological setup — vectors, reservoirs, susceptible populations — makes it a textbook candidate for wider emergence.
The virus is mobile. The world is changing. And now the question becomes — what happens when you meet Oropouche not in the lab or on a map, but in the body? What does it feel like to have it? How does it progress? That’s where we go next: the clinical story, symptom by symptom.
Clinical Manifestations: What It Feels Like When Oropouche Hits
So let’s say the bite happens. A tiny midge — you wouldn’t even notice it — finds a patch of exposed skin, maybe while you’re walking home in the evening or sitting by an open window. A few days go by. You feel fine. You probably forget the bite even happened. But then something changes.
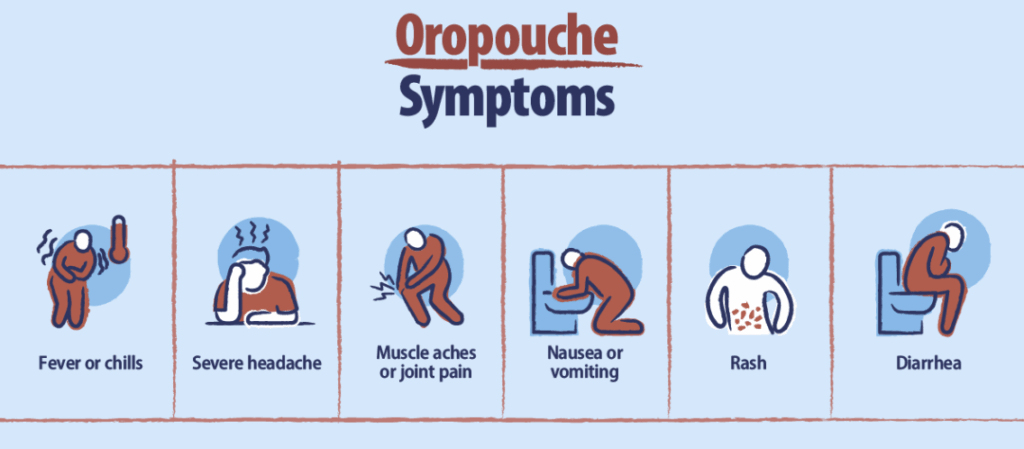
Usually around 4 to 8 days after exposure, it begins. A sudden wave of fatigue, out of proportion to what you’ve done that day. Maybe you write it off as poor sleep or dehydration. But then the fever sets in — not a mild, creeping warmth, but a full-body, temperature-spiking kind of fever that seems to arrive all at once. It comes with chills, and often a deeply uncomfortable sense of malaise — a medical word, yes, but in reality, it feels like your body has been unplugged from itself.
This is the classic onset of Oropouche Fever. The fever itself often lasts for about 3 to 6 days, and it’s usually accompanied by an assortment of other symptoms that aren’t specific enough to scream “Oropouche,” but are intense enough to keep you in bed. Headache is common, often described as frontal and pounding, sometimes retro-orbital — behind the eyes — a sensation that many dengue patients also report.
Then come the myalgias and arthralgias — muscle and joint pain — which can be sharp, migratory, or just a dull, widespread ache. Some people report feeling like they’ve been “run over,” with soreness that seems disproportionate to the fever itself. Photophobia (sensitivity to light) and dizziness can make sitting upright a challenge. And for some, a maculopapular rash — flat and raised red spots — appears a few days in, often on the trunk or limbs.
And then, for many people, it just… resolves. The fever fades. Appetite returns. The body feels like it’s been through a storm, but the skies are clearing. It’s tempting to move on.
But not everyone is so lucky.
There’s a catch with Oropouche that complicates the narrative: a biphasic illness can occur. Just when you think you’re done — maybe a day or two after the fever ends — symptoms can recur. A second wave. Not always as severe, but disorienting, especially for patients who thought they were recovering. Why it happens isn’t entirely clear, but it’s been documented in a significant subset of cases.
And then there are the rare — but very real — neurological complications. In some cases, the virus appears to cross into the central nervous system, triggering aseptic meningitis or even meningoencephalitis. These cases aren’t the majority, but they are medically significant. Patients present with high fever, neck stiffness, altered mental status, and in some cases, seizures or prolonged confusion. This adds a layer of clinical urgency to what might otherwise be dismissed as a self-limiting viral illness.
What’s frustrating is that there’s no clinical signature that’s uniquely Oropouche. It mimics dengue. It looks a lot like chikungunya. It feels like Zika, at least in the early phase. That’s why misdiagnosis is common — especially in areas where diagnostic tools are limited and co-circulating arboviruses are the norm. If you don’t test specifically for OROV, you might never know it was the culprit.
From a public health standpoint, this overlap in symptomatology is more than an inconvenience — it muddies the data. It hides outbreaks. It prevents clinicians from making targeted decisions. And it can lead to underreporting of neurological cases, which may require very different kinds of care and monitoring.
So, to sum it up — Oropouche isn’t usually deadly, but it’s debilitating. It’s confusing. It can recur, and in rare cases, it can invade the brain. It’s not the flu. It’s not a mosquito bite you forget about. It’s something else — and it deserves its own category in the clinician’s mind.
The next logical question is: how do we tell when it’s Oropouche and not something else? How do we confirm it, especially in places where resources are stretched thin? That’s where we go next — into the world of diagnostics, and why catching this virus early is harder than it should be.
Diagnosis: Knowing It’s Oropouche (When It Looks Like Everything Else)
Picture a crowded clinic somewhere in northern Brazil. It’s hot, it’s noisy, and the waiting room is full. Most of the patients are here with roughly the same set of complaints: fever, headaches, muscle pain, maybe a rash. The physician flips through case after case, trying to triage. Dengue? Possibly. Chikungunya? Maybe. But how do you spot Oropouche Fever in this crowd? That’s the puzzle.
Clinically, it’s a chameleon. Oropouche doesn’t wear a label. It walks and talks like every other arbovirus in the region — and that’s exactly what makes it so difficult to detect. The symptoms, while real and often intense, aren’t distinctive enough on their own. In the absence of specific testing, it’s a guessing game.
So, how do we diagnose it? The answer, unfortunately, is: it depends — on timing, technology, and geography.
In the first few days of illness, when the virus is still actively replicating in the blood, RT-PCR (reverse transcription polymerase chain reaction) is the gold standard. It’s sensitive, specific, and can detect the viral RNA directly. If you catch it during that window — usually within the first 4 to 6 days of symptoms — you’ve got a good shot at confirming OROV.
But here’s the snag: PCR testing for Oropouche isn’t widely available. In many endemic regions, labs are focused on dengue, Zika, and chikungunya — the “big three.” Oropouche testing is often only available through reference laboratories or research centers. That means patients might go undiagnosed, and outbreaks might smolder under the surface for weeks before anyone realizes what’s happening.
If PCR isn’t an option — or if the patient presents later in the course of illness — then we move into serology. ELISA tests can detect IgM antibodies produced in response to the virus, which typically appear around day 5 or 6. That’s useful, but not without pitfalls. IgM sticks around for weeks, sometimes months, and cross-reactivity with other viruses (especially in the Bunyavirus family) can blur the result. You might get a positive, but you won’t be sure what it’s really positive for.
To make matters more tangled, co-infections happen. A person could be infected with dengue and Oropouche at the same time. Or they might have had one virus recently and the other now — but the test won’t necessarily sort that out for you. It might just tell you that something’s there, and it might not be clear what.
There are more advanced options, of course — viral isolation in cell culture, next-generation sequencing, even plaque reduction neutralization tests (PRNT) — but these are largely confined to research labs. They’re not something a front-line clinician can rely on.
And then there’s the deeper problem: low clinical suspicion. In many places where OROV circulates, healthcare providers may not even think to test for it. It’s not because they’re careless — it’s because Oropouche still occupies a kind of gray space in public health messaging. It’s not notifiable in many countries. It’s not in the standard diagnostic workup. So unless someone has seen it before — or unless there’s an active outbreak with lab support — it often gets lumped into the “viral fever of unknown origin” category and quietly disappears from the data.
Which is exactly what makes surveillance so critical. If you don’t look for it, you won’t find it. And if you don’t find it, you can’t stop it.
What we need — and what’s slowly beginning to happen — is the development of rapid, point-of-care diagnostic toolsspecific to Oropouche. Something that can be deployed in the field, in rural clinics, in outbreak zones. Something that doesn’t require a centrifuge, a biosafety cabinet, or a week-long wait. Because if you can name it, you can start to manage it. You can identify patterns. You can see where it’s spreading next.
So the diagnosis of Oropouche isn’t just a matter of lab technique — it’s a race against time, access, and assumptions. And as we’ll see in the next part, once you do make the diagnosis, the question becomes: what then? What can we actually do for the patient? What treatments exist — or don’t? And what’s on the horizon? Let’s talk treatment next.
Treatment and Management: When There’s No Silver Bullet
Once you know a patient has Oropouche Fever — either through lab confirmation or sheer clinical intuition — the next question is the one you can’t avoid: what now? What do we do?
And here’s the honest, unglamorous answer: supportive care. That’s it. There’s no antiviral. No vaccine. No magic pill. The entire treatment protocol, as it stands today, is focused on making the patient more comfortable and letting their immune system do the heavy lifting.
So, in practice, that means treating the fever — paracetamol (acetaminophen) is usually preferred, because NSAIDs like ibuprofen can complicate things if the diagnosis is uncertain (especially in regions where dengue is also circulating). It means managing pain and muscle aches, encouraging hydration, and watching for red flags that suggest complications, especially neurological ones.
Most people recover fully. That’s the good news. For the vast majority of cases, the fever breaks, the body rebounds, and life resumes. But that doesn’t mean it’s an easy illness. The fatigue can linger for days or even weeks, and in areas with high transmission, people often get sick during the most economically critical parts of their year — planting season, harvest, fishing schedules. Recovery isn’t just biological; it has a social and economic cost.
And when complications do occur — meningitis or meningoencephalitis, for example — the approach shifts from simple fever control to hospital-based supportive care, possibly even intensive monitoring. Intravenous fluids. Anti-seizure medications. Neuroimaging if it’s available, though in most outbreak settings, it often isn’t. At that point, it’s about managing inflammation and preserving neurologic function. But even here, there are no virus-specific tools. We’re reacting, not preventing.
That brings up a tough reality. Why don’t we have an antiviral yet? Why isn’t there a vaccine? It’s a fair question — and it doesn’t have an easy answer. Part of it is visibility. Oropouche has spent decades flying under the radar, known mainly to scientists and public health workers in endemic areas. Funding tends to follow headlines, and Oropouche hasn’t made many — not yet.
Another part is technical. OROV belongs to a family of viruses that mutate quickly, and because it’s segmented, reassortment is always a risk. That makes vaccine development more complex, at least theoretically. But it’s not impossible. What’s missing, primarily, is momentum.
There are early-stage efforts underway, especially in Brazil. Researchers have been experimenting with DNA-based vaccines and recombinant viral vectors. Some animal studies have shown promise. But we’re still far from anything ready for mass use. As of 2025, there are no licensed vaccines for Oropouche. No specific antiviral agents in clinical trials. No monoclonal antibodies tailored to its glycoproteins.
Which means the best “treatment,” ironically, is not getting infected in the first place. That’s where prevention comes in — and we’ll get there in the next part. But for the moment, it’s important to sit with this truth: Oropouche is a virus we can’t yet fight directly. We can support the body, yes. We can ease the pain. We can recognize when things go south and intervene quickly. But we’re still playing catch-up.
That may not sound satisfying. It’s not supposed to be. Emerging diseases often outpace our toolbox — at least at first. But recognition is the first step. Once you understand the virus, you can start to work on controlling it. And that’s exactly where we’re headed next: how to stop it before it spreads.
Prevention and Control: Stopping Oropouche Before It Starts
If treatment is mostly supportive care and there’s no vaccine yet, then prevention becomes the frontline defense. But how do you prevent a virus that rides silently on tiny biting midges and mosquitoes — creatures that swarm at dusk, slip through cracks, and breed in puddles no bigger than a bottle cap?
First, you have to understand the enemy. Oropouche virus is, at its core, a vector-borne virus. That means interrupting transmission means interrupting the vector — those bloodsucking midges and mosquitoes. But here’s where things get complicated. The main culprit, Culicoides paraensis, is small and elusive, and unlike the Aedes aegypti mosquito — which breeds in larger, often obvious containers like tires and buckets — these midges breed in organic-rich environments like mud, decaying vegetation, and even drainage ditches. They’re active during the day and twilight hours, making avoidance tricky.
So, the first line of defense is personal protective measures: using insect repellents, wearing long sleeves and pants, and using physical barriers like fine mesh window screens or bed nets. But there’s a catch here, too — because these midges are so small, standard mosquito nets don’t always do the job. That means communities need access to better barriers and education about the limits of traditional methods.
At a broader level, environmental management is crucial. Reducing breeding sites by draining stagnant water or managing organic waste near homes can help, but it’s labor-intensive and requires community buy-in. It’s not as simple as spraying a few cans of insecticide. And speaking of insecticides, vector control programs are often underfunded and understaffed in the regions where Oropouche circulates. This isn’t just a problem of science — it’s a problem of resources and priorities.
Then there’s the challenge of urban versus sylvatic cycles. Because OROV exists both in wild animal reservoirs in the forest and in urban human populations, stopping it requires coordination between forest health and urban public health — two worlds that don’t always communicate well. When you clear forests or expand roads, you create more edges where humans, vectors, and animal reservoirs interact. It’s a perfect storm for spillover events.
Public health officials face the difficult task of early outbreak detection. If an Oropouche outbreak is caught early, community engagement becomes critical — educating people about symptoms, encouraging protective measures, and mobilizing healthcare resources to contain spread. But this requires surveillance infrastructure that’s often weak in rural and peri-urban areas.
Another prevention angle is travel advisories and screening. While Oropouche hasn’t yet made the jump to global pandemic status, its ability to cause explosive local outbreaks means that international travel could seed new areas. Monitoring travelers from endemic zones and educating them about protective measures can help slow this process.
So why isn’t there a big, coordinated Oropouche control program, like there is for malaria or dengue? It comes back to visibility and scale. Oropouche fever often gets overshadowed by more notorious arboviruses. Funding and attention follow the diseases that make headlines or cause massive deaths, which OROV usually doesn’t. But that’s a dangerous blind spot, because ignoring a virus like this until it becomes a crisis is exactly what history teaches us not to do.
In short, prevention and control of Oropouche are multifaceted challenges — involving vectors, environment, human behavior, and health systems. It’s not a single fix. It’s a mosaic of efforts, each piece necessary to keep this emerging virus at bay.
Next, we’ll explore the latest developments and breakthroughs — how research, technology, and surveillance are evolving in 2025 and beyond to give us new tools in this ongoing fight.
Recent Developments (2025–2026): What’s New in the Oropouche Story?
As we step into the present, it’s worth asking: what’s changed recently? Has Oropouche virus finally made its big move onto the global stage, or is it still lurking in the shadows, quietly shaping lives in tropical corners?
The answer is somewhere in between. Over the past couple of years, 2025 and 2026, Oropouche hasn’t exploded into worldwide headlines — yet — but it has definitely stepped up its profile among researchers, public health officials, and even local communities in endemic areas.
One of the most striking developments has been the improvement in diagnostic tools. Newer, portable RT-PCR kits tailored specifically for OROV are becoming more available in frontline clinics across parts of Brazil and Peru. These rapid molecular tests cut down the time to diagnosis from days or weeks to mere hours. Imagine how game-changing that is: catching outbreaks early, confirming cases more reliably, and distinguishing Oropouche from dengue or Zika quickly. It’s a critical step in closing the diagnostic gap that has long hampered effective responses.
And while a formal vaccine remains out of reach, the intensified research surrounding pan-arboviral platforms — especially those inspired by responses to novel bat-borne coronaviruses like HKU5-CoV-2 — offers cautious optimism that OROV may benefit from broader immunological strategies in the near future.
Beyond diagnostics, there have been notable outbreak investigations that have given scientists fresh insights. For example, a 2025 outbreak in the state of Pará, Brazil, involved over 1,500 confirmed cases, with a curious twist — researchers found evidence of genetic reassortment in the circulating strains. This means the virus swapped segments with related bunyaviruses, creating a slightly different version. Why does this matter? Because reassortment can affect how the virus behaves — its transmissibility, virulence, and immune evasion potential. It’s a vivid reminder that Oropouche isn’t a static target; it’s evolving right under our noses.
On the treatment front, while we’re still waiting for a licensed vaccine, there has been progress in vaccine research. Brazilian labs have advanced several candidate vaccines through preclinical trials, including DNA-based and viral vector platforms. Early results suggest promising immune responses in animal models, with researchers hopeful these candidates could move into human trials within the next couple of years. The road to a vaccine is long, but this momentum is encouraging.
In the realm of public health policy, some countries have started to formally include Oropouche in their arboviral surveillance systems. That’s huge. Official recognition means better data collection, more funding, and targeted public health messaging. Brazil, for instance, has rolled out pilot programs to train healthcare workers specifically on Oropouche identification and management in high-risk areas. These programs aim to close the knowledge gap that has kept the virus underdiagnosed.
Another area of recent focus has been on vector ecology. Advances in remote sensing and environmental DNA (eDNA) sampling are helping scientists map midge populations and identify breeding hotspots with unprecedented precision. This information feeds into predictive models, helping public health teams anticipate outbreaks before they start — a kind of early warning system.
But not all news is positive. Climate change continues to push the boundaries of vector habitats. The rainy seasons have become more erratic, creating new breeding sites, while urban expansion continues to blur the line between forest and city. In 2026, there were reports of suspected Oropouche cases cropping up in areas of Central America where the virus hadn’t been documented before. Confirmations are still pending, but if true, it signals a geographic leap that merits close watch.
So, while Oropouche virus hasn’t yet claimed global attention, the pieces are moving fast. Scientific tools are sharper, surveillance is better, and the virus itself is showing signs of genetic agility. It’s a moment of both opportunity and caution — an opportunity to get ahead of this emerging threat, and a cautionary note that complacency could cost dearly.
Next up: pulling all this together. What does it mean for the future? How do we prepare? That’s where we go in our final section.
Oropouche Fever: Frequently Asked Questions (FAQ)
What is Oropouche virus and why is it important?
Oropouche virus (OROV) is an arbovirus transmitted mainly by biting midges (Culicoides paraensis) and mosquitoes, causing Oropouche Fever—an illness common in parts of South and Central America. It’s important because it can cause large outbreaks, often mistaken for dengue or other tropical fevers, and it has potential to spread as environments and vector habitats change.
Where is Oropouche virus found?
Primarily in northern and central South America, including Brazil, Peru, Panama, and Trinidad and Tobago. Recent evidence suggests it might be moving into new regions in Central America due to environmental changes.
How does Oropouche virus infect people?
People are infected when bitten by infected midges or mosquitoes that have picked up the virus from animal reservoirs or humans. The virus enters the bloodstream and can cause systemic symptoms.
What are the common symptoms of Oropouche Fever?
Symptoms usually include sudden high fever, headache, muscle and joint pain, dizziness, rash, and sometimes photophobia. In some cases, symptoms can recur after initial improvement.
Can Oropouche virus cause serious complications?
Yes. Although most cases are mild and self-limiting, in rare instances, the virus can invade the central nervous system causing meningitis or meningoencephalitis, which require urgent medical attention.
How is Oropouche Fever diagnosed?
Diagnosis is primarily through laboratory tests such as RT-PCR during the early stage of infection or serological tests detecting antibodies later on. However, limited availability of these tests and symptom overlap with other arboviruses often delay diagnosis.
Is there a vaccine or specific treatment for Oropouche Fever?
No vaccine or specific antiviral treatment is currently available. Management focuses on supportive care — relieving fever, pain, and maintaining hydration.
How can Oropouche virus infection be prevented?
Prevention relies on avoiding bites from midges and mosquitoes using repellents, protective clothing, bed nets (though midges can be very small), environmental management to reduce breeding sites, and vector control efforts.
Why is Oropouche virus considered an emerging threat?
Changes in climate, urban expansion, increased travel, and the virus’s ability to reassort genetically all contribute to its potential to cause larger, more frequent outbreaks and possibly spread beyond traditional regions.
What recent advances have been made in Oropouche research?
Recent developments include improved rapid diagnostic tests, early-stage vaccine candidates progressing in trials, expanded surveillance programs, and better understanding of vector ecology and viral genetics.
Why is Oropouche Fever often underreported or misdiagnosed?
Because its symptoms closely resemble other arboviral diseases common in the same regions, and because testing resources specific to OROV are scarce, many cases go unrecognized or are attributed to other viruses.
What is being done to improve public health responses to Oropouche outbreaks?
Some countries have started integrating Oropouche surveillance into existing arboviral monitoring systems, training healthcare providers, and investing in community education and vector control strategies to better detect and contain outbreaks.
Final Thought
Oropouche Fever may still be unfamiliar to many, but it embodies the challenges of emerging infectious diseases in our rapidly changing world. It’s a reminder that viruses don’t wait for us to be ready—they adapt, spread, and sometimes surprise us. What makes Oropouche especially compelling is how it quietly moves between forests and cities, slipping under the radar while impacting thousands.
The story of Oropouche isn’t just about a virus; it’s about the interplay between humans, animals, and the environment—and how that balance shapes global health. It teaches us the importance of vigilance, investment in diagnostics and research, and the need for coordinated action before small threats become big problems.
Understanding Oropouche today means being better prepared for the unknown viruses of tomorrow. In that way, the lessons it offers extend far beyond the tropics—into every community where the next emerging infection might take hold. Paying attention now isn’t just smart; it’s necessary.
Because in the world of infectious disease, the quiet ones often demand the most attention.










