Nipah Virus: A Zoonotic Threat with Pandemic Potential
Introduction
What is the Nipah virus, and why are we talking about it now?
The Nipah virus—often shortened to NiV—isn’t the kind of pathogen you hear about on the evening news every day. It doesn’t cause massive annual waves of illness like influenza, and it hasn’t yet sparked a global shutdown like COVID-19. So why does it keep showing up on the World Health Organization’s list of priority pathogens? Why is it on the radar of virologists, epidemiologists, and global health officials?
Because it’s a textbook example of what public health experts call an emerging zoonotic threat—a virus that jumps from animals to humans and has the potential to cause widespread outbreaks. Think SARS. Think MERS. Think COVID-19. Nipah fits the same mold, but with a chilling difference: its case fatality rate can be as high as 75% in some outbreaks. That means three out of four people who get it don’t survive.
Where did it come from—and how did it first catch our attention?
The virus was first identified in 1998, in a small town called Sungai Nipah in Malaysia. Farmers started noticing something strange: pigs on their farms were showing signs of severe respiratory illness, and soon after, the farmers themselves began falling seriously ill—some with fever and confusion, others with seizures or coma. The connection wasn’t immediately clear, but by the time the dust settled, over 100 people had died, and millions of pigs had to be culled to stop the outbreak.

The culprit? A virus that had jumped from fruit bats—the natural reservoir—to pigs, and then to humans. That was the first recorded outbreak, but not the last.
Why does it matter in the bigger picture of public health?
Since that first event, recurrent outbreaks have occurred in Bangladesh, India, and potentially other parts of South and Southeast Asia. These outbreaks tend to follow a disturbing pattern: small clusters of deadly illness, often beginning with contact with bats or contaminated fruit, then spreading to caregivers or healthcare workers. The virus is capable of direct human-to-human transmission, particularly through close contact, which makes it especially dangerous in settings where infection control is limited.
And here’s the key point: NiV isn’t just dangerous because of what it has done—it’s dangerous because of what it could do. The virus has shown it can adapt. It has already expanded beyond a single species jump. And if it ever mutates to become more easily transmissible between humans—like through the air or via casual contact—it could be devastating.
Pathogens like Nipah don’t exist in a vacuum. They’re part of a broader ecosystem of emerging viral threats—many of which share eerie parallels in terms of their origins and impact. Marburg virus, for instance, also emerged from wildlife reservoirs and has caused deadly outbreaks under the radar. It’s a useful comparison point when thinking about how these diseases jump species and catch us off guard.
So where are we now?
As of 2025, we’re in a strange in-between moment with Nipah. We’re aware of it. We’re watching it. But in many ways, we’re still playing catch-up. We have no licensed vaccine. No approved antiviral treatments. Diagnostic tools exist, but they’re not widespread or especially fast. Outbreak responses often come after the fact, when people are already sick—or dying.
Which leads to the uncomfortable truth: Nipah virus is a looming threat that we might not be ready for. That’s why understanding it—its origins, its biology, its impact—isn’t just a scientific exercise. It’s a matter of future preparedness.
Virology and Pathogenesis
Let’s dig into the virus itself. What is Nipah made of—and how does it work?
If you could zoom in with an impossibly powerful microscope and take a look at Nipah virus up close, you’d find something that seems deceptively simple. It’s just a strand of single-stranded, negative-sense RNA, wrapped in a lipid envelope. No cell wall. No nucleus. No independent metabolism. On paper, it looks harmless. But like many viruses, simplicity is its greatest weapon.
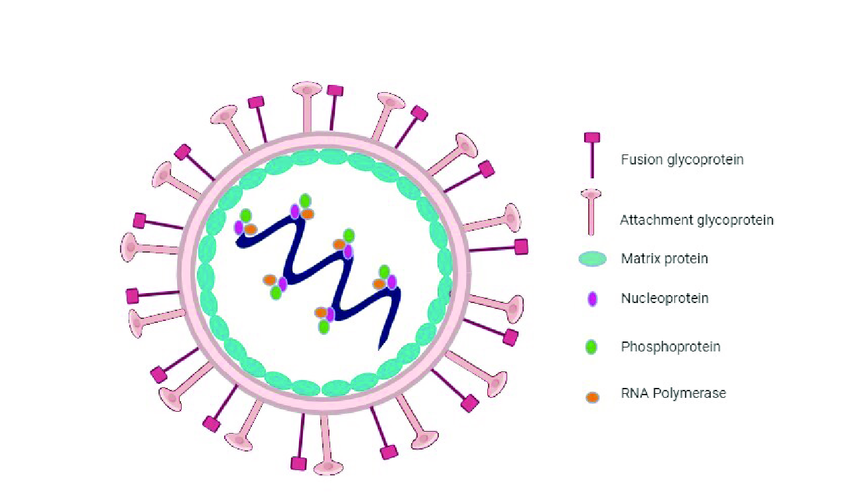
NiV is part of the Henipavirus genus, which also includes the Hendra virus. These viruses belong to the Paramyxoviridae family, which you might know because it includes more familiar names like measles and mumps. But unlike those, Nipah isn’t content with just infecting the respiratory system—it has a much broader range of targets, including the central nervous system, which makes it uniquely devastating.
What’s the structure like—and why does it matter?
On the outside of the virus are two key proteins: the G (glycoprotein) and the F (fusion) protein. Think of them as the virus’s lock-picking tools. The G protein binds to receptors on human cells—specifically ephrin-B2 and ephrin-B3, which are found in high concentrations in the brain, lungs, and endothelial tissues. Once the virus latches on, the F protein helps it fuse with the host cell membrane, opening the door so its RNA can slip inside.
This is what allows the virus to enter not just respiratory cells (like many airborne viruses), but also neurons and blood vessel linings, explaining its unique ability to cause both pneumonia and encephalitis in the same patient.
What happens once it’s inside a human cell?
Once the RNA genome is released into the host cell, it hijacks the cell’s machinery to make copies of itself—proteins, RNA strands, and eventually full virus particles. This replication is fast, aggressive, and—unfortunately—quite efficient. The infected cell is essentially turned into a virus factory, cranking out new particles until it either bursts or dies from the burden.
But the story doesn’t end there. Nipah also causes a massive immune response. In many cases, this response becomes overactive—leading to inflammation that contributes to tissue damage. In the brain, this can cause brain swelling, seizures, and coma. In the lungs, it can result in acute respiratory distress, making breathing difficult or impossible.
How does NiV compare to other viruses in terms of pathogenesis?
In terms of severity and complexity, it sits somewhere between rabies and Ebola. Like rabies, it has a neurotropic component—meaning it loves the nervous system. Like Ebola, it can trigger multi-organ failure. But NiV brings something extra to the table: silent spread in the early stages, during which symptoms may be mild or nonspecific. This can delay diagnosis and isolation, increasing the risk of further transmission.
And there’s another insidious feature: viral persistence. In some survivors, the virus has been found to linger in immune-privileged sites like the brain, potentially reactivating months or even years later. That makes long-term monitoring crucial—and underscores how little we still know about this pathogen.
Why is understanding the virology so important?
Because every aspect of how this virus functions tells us something about how to fight it. If we know how it enters cells, we can block those receptors. If we know what triggers its replication, we can design drugs to interrupt that process. If we understand its preference for brain tissue, we can predict complications early.
In other words, virology isn’t just academic—it’s the foundation of every diagnostic test, every treatment plan, and every vaccine candidate being developed right now.
Epidemiology
Where in the world is the Nipah virus found—and why does it seem to keep coming back?
You might think a virus this deadly would be easy to track, but Nipah isn’t spread evenly across the globe. Instead, it appears in hotspots, mostly in parts of South and Southeast Asia. The main countries that have reported confirmed outbreaks are Malaysia, Bangladesh, and India—though the virus’s natural host, fruit bats of the Pteropus genus, are found much more broadly, stretching from East Africa to Australia.
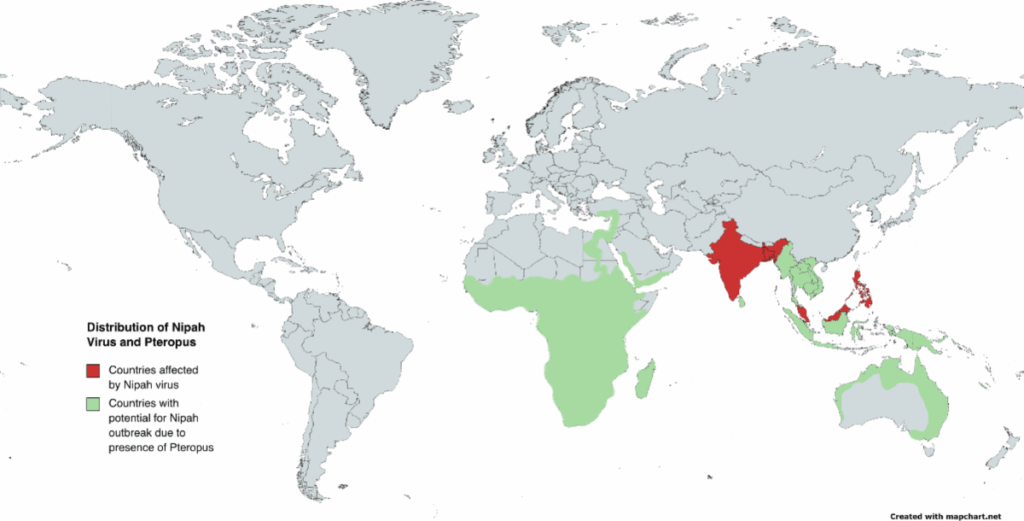
So the virus has the geography. It has the host. The real question is: what makes some places break out in human cases while others don’t?
How does Nipah virus jump from animals to humans?
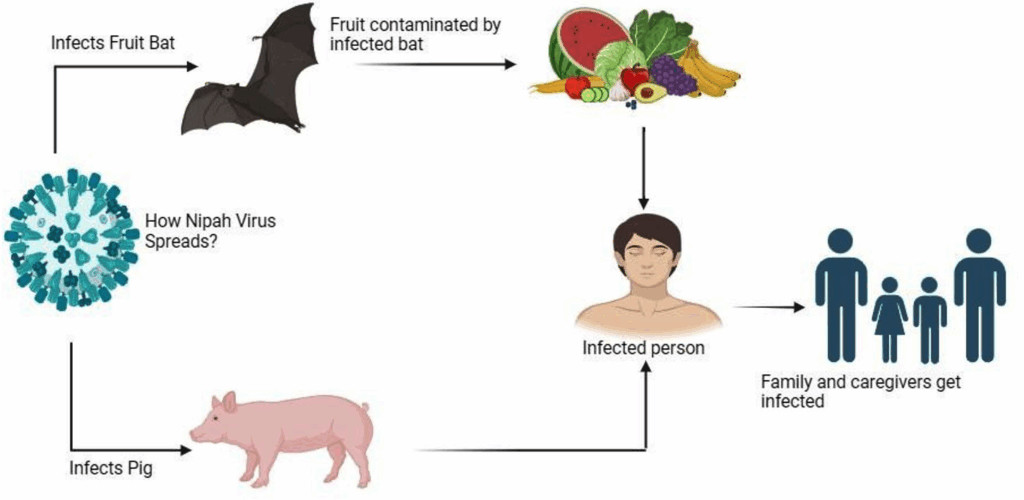
This is where things get really interesting—and a bit unsettling. The virus doesn’t usually start in humans. It starts in fruit bats, often called flying foxes. These bats are completely unaffected by the virus; they don’t get sick. But they shed the virus in their saliva, urine, and feces, which means they can contaminate the environment wherever they roost or feed.
One classic route of spillover is through date palm sap—a traditional and popular drink in parts of rural Bangladesh and India. Bats like the sugary sap and will often lick or urinate into the collection pots left on trees overnight. People drink the raw sap in the morning, unaware that it might now be laced with virus.
Another pathway? Infected pigs. That’s how the first outbreak in Malaysia started. Bats dropped fruit into pig pens, pigs ate the contaminated fruit, and then farmers got sick from close contact with their livestock. It was a chain reaction: bat → pig → human.
Is it just an animal problem, then? Or can people spread it too?
This is the part that moves Nipah from a wildlife curiosity to a public health threat. Yes—humans can and do transmit the virus to each other. In fact, in some outbreaks in Bangladesh and India, a significant proportion of cases came not from contact with animals but from contact with infected people—especially during the late stages of illness when respiratory symptoms are severe and viral shedding is high.
Transmission can happen through:
- Respiratory droplets (from coughing or sneezing)
- Contaminated body fluids (like saliva, urine, or even sweat)
- Close physical contact—especially in caregiving situations without proper protection
This human-to-human spread has been limited in scale so far, but that doesn’t mean it will stay that way. Every time the virus infects a new host, it gets another chance to evolve. That’s why epidemiologists keep a close eye on it.
Who is most at risk?
Typically, the highest risk groups are:
- People living in rural or forest-adjacent areas, especially those who collect or consume raw date palm sap
- Farmers and animal handlers, particularly during pig or livestock outbreaks
- Healthcare workers, due to close contact with patients and sometimes limited access to protective gear
- Family members or caregivers who tend to the sick at home
In some outbreaks, entire clusters of cases have emerged within a single household or village—something that adds to the sense of unpredictability.
Are there seasonal patterns—or is this virus just random?
There actually does seem to be a seasonal trend, particularly in Bangladesh, where most outbreaks occur in the winter and early spring. That coincides with the peak season for date palm sap harvesting—and therefore, higher chances of bat-human contact. But in other regions, such as Kerala in southern India, outbreaks have shown up in different months, suggesting that environmental and social factors both play a role.
Is the virus spreading to new areas? Should we be worried about that?
There’s no large-scale geographic expansion of confirmed human cases yet, but the potential is very real. The bat hosts are highly mobile, and they’re not restricted by borders. Habitat destruction, climate change, and human encroachment into wild areas are pushing bat populations into closer contact with humans and domestic animals. That means new spillover events could happen anywhere the right conditions line up.
In fact, some serological surveys have found evidence of henipavirus exposure in bats far outside the known outbreak zones—in parts of Africa and the Pacific. That raises a sobering possibility: the virus might be more widespread than we realize; we just haven’t looked hard enough.
So what’s the bottom line on Nipah’s epidemiology?
Nipah isn’t just a local concern. It’s a regional threat with global implications. It’s a virus that can jump from bats to humans via food, to humans via animals, and then from person to person. It appears sporadically, kills quickly, and disappears again—until the next time. That makes it hard to study, hard to predict, and very hard to prepare for. But it also makes understanding its transmission dynamics absolutely essential if we hope to stay ahead of the next outbreak.
Clinical Manifestations
What does it actually feel like to get Nipah virus? What happens inside the body once someone’s infected? These are the kinds of questions that haunt clinicians, especially during outbreaks where the virus seems to move differently from one person to the next. And unlike many familiar illnesses, Nipah doesn’t have a single, predictable presentation. It can feel like a flu, it can look like pneumonia, and then—without warning—it can turn into a full-blown neurological crisis. That unpredictability is part of what makes the virus so dangerous.

The early phase of the illness is often frustratingly ordinary. A person might feel feverish, fatigued, maybe a little dizzy or sore. There’s often a headache—sometimes mild, sometimes pounding—but nothing at first that screams “deadly virus.” Some people report muscle aches or nausea, and a few develop a cough or sore throat. All of these could easily be brushed off as seasonal flu or some viral fatigue. And that’s precisely why early cases can go unnoticed, especially in places where diagnostic capacity is limited. People don’t race to the hospital for a headache. Doctors don’t immediately test for Nipah in someone with a fever and general malaise.
But then things can change—and change quickly. In a worrying number of cases, those subtle symptoms escalate into confusion, disorientation, and eventually, a breakdown of basic neurological functions. The virus, we now know, has a marked preference for the brain. It causes acute encephalitis—inflammation of brain tissue—which leads to seizures, coma, and often death. It’s not a slow deterioration, either. In many documented outbreaks, patients have gone from being alert to unresponsive in a matter of hours. And because this kind of rapid encephalitis is rare in most viral illnesses, it often catches health workers by surprise.
Respiratory symptoms may also develop in parallel—or even precede the neurological ones. In some outbreaks, particularly those involving human-to-human transmission, the virus seems to take a more pulmonary route. People report difficulty breathing, persistent cough, low oxygen levels, and in severe cases, respiratory failure. This dual tropism—meaning the virus targets both the respiratory system and the central nervous system—makes it especially lethal and difficult to manage in clinical settings. One minute you’re treating a patient with pneumonia; the next, you’re watching that same patient lose consciousness due to brain inflammation. And if you don’t know what you’re dealing with, that transition can be missed entirely until it’s too late.
It doesn’t stop there. In some patients, particularly those who survive the acute infection, the consequences linger long after the virus has cleared from the bloodstream. Neurological damage can be permanent. Survivors have reported memory loss, mood changes, motor dysfunction, and chronic fatigue. There’s even the rare but chilling phenomenon of relapsing encephalitis, where the virus reactivates months or even years after recovery, causing a second wave of brain inflammation that may be even more devastating than the first. Why this happens in some individuals and not others is still unclear, but it highlights just how little we understand about Nipah’s long-term effects.
Mortality rates vary by region and by outbreak, but they are almost always high—sometimes alarmingly so. In Malaysia’s 1998 outbreak, the case fatality rate was around 40 percent. In Bangladesh and India, some outbreaks have seen death rates approach or exceed 70 percent. These are not theoretical numbers. They represent real people—fathers, daughters, caregivers—often dying within days of symptom onset. And because of the limited treatment options, the outcome depends heavily on the speed of diagnosis and the quality of supportive care available.
There have been rare cases of people testing positive for Nipah virus without developing any symptoms at all. These asymptomatic cases are important from a public health perspective because they suggest a potential for silent transmission, although that link hasn’t been firmly established yet. What we do know is that most infections don’t go unnoticed. When Nipah strikes, it does so with force, and it often leaves devastation in its wake.
The clinical picture of Nipah is, in essence, a spectrum. At one end, you have mild cases that might pass under the radar. At the other, you have fulminant encephalitis and respiratory collapse. In the middle lies a range of overlapping symptoms that challenge our ability to respond quickly. And because the virus attacks so many systems—lungs, brain, sometimes even the heart—it’s not just one disease we’re dealing with. It’s several, rolled into one.
That’s why understanding the clinical manifestations of Nipah isn’t just academic. It’s the foundation of everything from outbreak triage to long-term care. It tells us who to test, when to act, and how to prepare for what’s coming—because when the symptoms show up, we may already be behind.
Diagnosis
How do you diagnose a virus that can mimic the flu one day and cause fatal brain swelling the next? That’s the challenge with Nipah. Early detection is both critical and notoriously difficult, because in the initial stages, there’s nothing distinctly “Nipah” about the symptoms. A fever, a headache, maybe some fatigue—those are signs of a dozen other, far more common illnesses. That means clinicians in outbreak-prone regions have to walk a fine line between caution and overreaction. But the cost of missing a case is steep.
The gold standard for diagnosing Nipah virus is real-time reverse transcription polymerase chain reaction—RT-PCR. It’s a lab technique that identifies and amplifies the viral RNA, essentially “catching” the virus in action. The test is usually performed on body fluids such as throat swabs, nasal secretions, cerebrospinal fluid, or even urine. If done properly and early enough, it’s highly accurate. But here’s the catch: you need the equipment, you need trained staff, and you need the right samples—taken at the right time. And that’s not a given in rural areas where outbreaks often start. In fact, in many cases, by the time a sample is collected and sent to a central lab, the patient’s condition may have already deteriorated—or the opportunity to prevent transmission may have passed.
There’s also ELISA, a type of serological test that detects antibodies—either IgM or IgG—produced in response to the virus. This can help confirm a diagnosis in the later stages of illness or in retrospective investigations, especially when trying to understand the true spread of an outbreak. But again, the problem is timing. Antibodies don’t appear immediately. If someone is acutely ill and the test comes back negative, it may not be that they’re uninfected—it may simply be too soon.
One of the hardest parts of managing Nipah isn’t just the speed—it’s the subtlety. It hides behind symptoms that look like everything and nothing at once. That’s why foundational tools—like complete blood counts and broader diagnostic panels—can quietly become critical. Not just for spotting cancer or chronic conditions, but for flagging unusual patterns that hint at something new, something viral. The infrastructure we build for routine diagnostics often ends up doing double duty during outbreaks.
Another method that’s occasionally used is virus isolation, where the pathogen is grown in a lab from a patient’s sample. This is the most definitive diagnostic technique available—but it’s also dangerous, expensive, and slow. It requires a biosafety level 4 (BSL-4) facility, of which there are very few in the world. So while virus isolation is useful for research and confirmation, it’s not a frontline tool during an active outbreak.
One of the biggest challenges in diagnosing Nipah is exactly that: speed. In a fast-moving outbreak, waiting for lab results—sometimes days away—can cost lives. That’s why there’s an urgent push for more rapid, field-deployable diagnostic tools. In recent years, there’s been promising work on portable PCR machines and antigen-based rapid tests. These could, in theory, allow health workers in remote locations to screen patients within minutes rather than days. But widespread deployment is still in the works.
Another obstacle is the clinical overlap with other diseases. A patient might show up with fever and confusion during dengue season, or a respiratory infection that looks like pneumonia in a tuberculosis-endemic region. Without a strong reason to suspect Nipah—and without a confirmed link to an ongoing outbreak—health workers may not even test for it. And that delay in clinical suspicion is a huge blind spot. It’s not that the tools don’t exist; it’s that the awareness and infrastructure to use them at the right moment aren’t always in place.
On top of that, there’s the human element. During outbreaks, fear and stigma can make people avoid clinics altogether. Family members may not report symptoms right away, and patients may hide recent travel or exposure history. This adds yet another layer of uncertainty in an already complex diagnostic puzzle.
In short, diagnosing Nipah is not just a scientific or technical issue—it’s a logistical, psychological, and systemic challenge. We have good tests, but we need to make them faster, cheaper, and more accessible. We need to train frontline workers not just to collect samples but to think about Nipah when faced with unusual or rapidly progressing symptoms. And we need public health systems that can respond quickly to suspected cases—not in weeks, but in hours.
Because with a virus this deadly, every hour counts.
Treatment and Management
So what do you actually do when someone is diagnosed with Nipah virus? What does treatment even look like for a disease that moves so fast and kills so many? The short answer—and it’s not a reassuring one—is that there’s no cure. No magic pill. No antiviral drug that can reliably stop the virus in its tracks. As of now, everything we do for a patient with Nipah falls under the broad and often frustrating category of supportive care.
Supportive care sounds vague, and in a way, it is. It’s not about killing the virus—it’s about keeping the patient alive long enough for their body to try to fight it off. This means maintaining hydration, managing fever, keeping the airways open, and if necessary, providing mechanical ventilation. In patients with neurological symptoms, that might mean controlling seizures, reducing brain swelling, and carefully monitoring vital signs. The idea is to buy time—stabilize the body, reduce complications, and hope the immune system can do the rest.
In theory, this should work. In practice, though, it depends heavily on when the patient shows up and what resources are available. In rural clinics with limited access to intensive care units, ventilators, or even consistent electricity, “supportive care” becomes a much more limited concept. And given how fast Nipah can progress—sometimes going from mild confusion to coma in less than 48 hours—those limitations can make the difference between life and death.
But surely, you might ask, with all the global focus on pandemics, there must be some drugs in the pipeline? And yes—there are. A handful of experimental therapies have shown promise in preclinical studies and, more recently, in compassionate use cases during outbreaks. One of the most closely watched candidates is a monoclonal antibody called m102.4, originally developed against the Hendra virus, a close cousin of Nipah. This antibody has been tested in animals with good results and even administered to some humans under emergency use protocols. The theory is that it binds to the virus’s surface proteins and neutralizes it before it can infect cells. It’s not yet a cure, and it’s far from being widely available, but it’s a glimmer of hope.
There’s also been exploration into using antiviral agents like ribavirin, a broad-spectrum drug sometimes used against other RNA viruses. During the 2001 outbreak in India, some patients were treated with ribavirin, and anecdotal reports suggested a possible benefit. But the evidence is thin, and controlled trials are lacking. Ribavirin may reduce mortality, or it may not. The truth is, we still don’t know.
Then there’s the question of vaccines—because prevention, in many ways, is the ideal form of treatment. Vaccine development has ramped up in recent years, especially following COVID-19, which proved that with enough urgency and coordination, vaccines for emerging viruses can be developed in record time. Several Nipah vaccine candidates are now in various stages of development. One of the most advanced uses a viral vector platform—essentially inserting a gene from the Nipah virus into a harmless carrier virus, which then trains the immune system to recognize and fight the real thing. Animal trials have been encouraging. Human trials have started. But again, access is limited, and approval is still years away.
Even if a vaccine were available tomorrow, distribution would face the same challenges seen with other epidemic diseases: logistical hurdles, public acceptance, regulatory approval, and of course, funding. And unlike COVID-19, which triggered a global mobilization, Nipah hasn’t yet inspired that kind of urgency—because, so far, its outbreaks have been small, contained, and geographically limited. That’s a dangerous form of complacency.
Management also means managing the context—the environment, the contacts, the community. For every confirmed case, there are usually dozens of exposed individuals who need to be monitored, tested, or quarantined. That’s a logistical nightmare in densely populated areas with limited health infrastructure. Contact tracing becomes essential, but also exhausting. And healthcare workers—often the first exposed and the first infected—need to be protected through proper training, adequate personal protective equipment, and clear protocols. Without these, hospitals themselves can become epicenters of transmission, as has happened in past outbreaks.
So where does that leave us? Right now, treating Nipah is less about fighting the virus and more about managing its consequences. It’s about buying time, reducing damage, and hoping that the body can hold the line. It’s about making the most of limited tools, while pushing urgently for better ones. And it’s about learning—every time there’s an outbreak—what worked, what didn’t, and how to do better the next time. Because with a virus like this, there will be a next time.
Prevention and Control
If we can’t cure Nipah virus, and we don’t yet have a widely available vaccine, the natural next question is: how do we stop it? What’s the strategy when your best medical tool is avoidance?
The truth is, when it comes to Nipah, prevention doesn’t start in a hospital. It starts in the forest, on farms, in food markets, and in places where people and animals interact in increasingly complex ways. Because this is, at its core, a zoonotic disease—one that begins in animals and crosses over into humans under the right (or wrong) conditions. So the first line of defense isn’t a pharmaceutical—it’s understanding and reshaping the interface between humans and the natural world.
The natural reservoir of Nipah is the fruit bat, particularly the species in the Pteropus genus. These bats aren’t villains. They’re ecologically important, and in their own systems, they carry the virus without suffering from it. But as forests are cleared for agriculture and urban expansion, bat habitats are disrupted, forcing them into closer proximity with people. They forage in orchards, they nest near farms, they drink from the same water sources as livestock. This shift in behavior isn’t random—it’s driven by us. So one of the most critical forms of prevention is ecological: protecting natural bat habitats to reduce human-wildlife friction in the first place.
Then there’s the matter of food safety, which in some outbreak regions turns out to be more important than most people realize. In Bangladesh, for example, drinking raw date palm sap is a deeply ingrained cultural tradition. The problem? Fruit bats love it too. They’re often seen lapping it up at night, contaminating the collection jars with saliva or urine. So the sap ends up carrying more than sugar—it may carry Nipah virus. Public health campaigns have tried to address this, introducing simple interventions like bamboo skirts or covers over the jars to keep bats away. It’s not high-tech, but it works—when people use it. And that’s the challenge. Prevention depends not just on knowledge but on adoption. You can’t enforce these changes from a distance. They have to be rooted in local engagement, built on trust, and responsive to the social meaning of food, tradition, and livelihood.
When outbreaks do occur, the prevention strategy shifts gears into containment. That’s where rapid response becomes everything. The goal is to isolate cases, trace contacts, and prevent the virus from moving beyond the initial cluster. But this is easier said than done. In many places where Nipah has emerged, health systems are already stretched thin. Rural clinics may lack isolation wards. Surveillance systems may not have the speed or coverage to catch early transmission chains. And even when the systems are in place, stigma and fear can delay reporting. People don’t want to be quarantined. Families are afraid to lose loved ones to isolation as well as disease. These human responses are understandable—but they’re also part of the risk landscape.
For healthcare workers, prevention takes on a very personal meaning. In some outbreaks, they’ve been among the first to be infected, and the first to die. That’s why infection prevention and control—what’s known in the medical world as IPC—becomes a frontline tool. It means using protective equipment correctly, following strict hygiene protocols, safely disposing of waste, and minimizing invasive procedures unless absolutely necessary. But again, this requires training, resources, and reinforcement. You can’t hand someone a face shield and expect miracles. They need to know when and how to use it—and they need to trust that the system has their back.
At the community level, public health messaging plays an enormous role. And not just during outbreaks. Educating people about transmission risks, safe caregiving, the symptoms to watch for, and the importance of early reporting builds a kind of social immunity. The more people understand what Nipah is, and how it spreads, the more likely they are to take precautions that matter. But messaging is only effective when it’s tailored. A generic pamphlet doesn’t change behavior. What works is local language, cultural context, and voices that people already know and trust.
There’s also a broader kind of prevention that operates on a national and international scale: surveillance. This means actively monitoring for signs of spillover—sampling bat populations, tracking respiratory illness trends in humans, keeping an eye on unexplained neurological syndromes. Surveillance isn’t glamorous, and it doesn’t make headlines, but it’s how we catch emerging threats early. When it works well, it can stop an outbreak before it begins. When it fails—or when it’s underfunded or ignored—Nipah has room to move.
Ultimately, preventing and controlling Nipah virus requires coordination at every level: from forest conservation efforts to family caregiving decisions, from village health workers to global research institutions. It’s a puzzle with ecological, medical, social, and political pieces. And it demands a kind of humility too. Because this isn’t a virus we can bully into submission. We have to work around it, anticipate it, and prepare for it—not just react when it’s already at our door.
Recent Developments (2025–2026)
Has anything changed lately? Are we any closer to understanding Nipah—or stopping it? That’s the natural question to ask, especially after years of watching new outbreaks flare up and die down, never quite becoming a global crisis but never going away either. And in the last year or two, the answer is cautiously optimistic. Things are starting to shift.
One of the biggest developments in the past year was the outbreak in southern India in late 2025. It wasn’t the largest on record—only about two dozen confirmed cases—but it grabbed international attention for one specific reason: asymptomatic transmission was suspected. For the first time, health officials noticed that a small number of secondary infections occurred in people who had no direct contact with the patient during the severe phase of illness. That raised an uncomfortable question: could the virus be spreading before symptoms appear? Until now, most Nipah transmission was thought to occur during late-stage illness, when patients were coughing or in close contact with caregivers. But if people are infectious earlier—or worse, while still feeling fine—that changes the playbook. It would mean our current containment strategies, which focus heavily on isolating the visibly sick, might not be enough.
This outbreak also prompted another wave of diagnostic innovation. Several biotech teams, working with global partners, pushed forward portable diagnostic devices—the kind that don’t need a full laboratory or cold storage. One such tool, a field-deployable rapid PCR kit, was successfully piloted during the Indian outbreak. It allowed frontline workers to test suspected cases on-site and get results within an hour. That’s a game-changer in regions where waiting days for a centralized lab result could mean an entire village is exposed. While these devices are still limited in number and cost, they signal a shift toward more accessible, real-time surveillance—something the Nipah response has sorely lacked for years.
Another area of movement is in vaccine development, which has picked up momentum in the post-COVID research landscape. One candidate, a recombinant vesicular stomatitis virus (rVSV)-based platform—similar in design to the Ebola vaccine—entered Phase II trials in early 2026. This is significant not just scientifically but psychologically. Until now, Nipah vaccine work lived in the shadow of larger, more immediate threats. But seeing it advance into human trials means it’s no longer just a “research topic.” It’s now a pipeline priority. There’s still a long road ahead before any Nipah vaccine becomes available for routine use—questions around efficacy, dosing, long-term immunity, and logistics remain—but the forward motion is real.
Therapeutically, there’s been continued interest in monoclonal antibodies. m102.4, the most promising candidate for years, finally received emergency use authorization in outbreak regions. This doesn’t mean it’s a standard treatment yet, but it does mean it can be deployed under controlled circumstances during flare-ups. Several patients in the 2025 India outbreak were given the antibody cocktail as part of a post-exposure protocol. The outcomes were mixed—not a miracle cure, but some evidence of shortened disease course in early-stage patients. More data is being collected, and follow-up studies are underway.
We’re also seeing more investment in ecological surveillance. International teams are partnering with regional governments to track bat migration patterns and conduct environmental sampling in high-risk zones. This isn’t just about knowing where bats live—it’s about understanding how their movement intersects with human activity, especially during fruiting seasons or climate disruptions. Satellite data is being used to model potential spillover zones, creating “risk maps” that could inform future prevention strategies.
And perhaps just as importantly, we’re seeing cultural adaptation. In rural Bangladesh, where raw date palm sap was once a seasonal staple, local cooperatives are now producing heat-treated sap drinks—safe from contamination, but still culturally resonant. In Kerala, where public trust was shaken during earlier outbreaks, health officials held community listening sessions to co-create outbreak response plans. These may seem like small changes, but they represent something larger: a shift from top-down interventions to community-led resilience.
If there’s a thread running through all these outbreaks—from Nipah to others—it’s that most of them start in silence. They spread slowly at first, often overlooked, until suddenly they’re not. Avian Influenza H5N1 followed a similar arc: localized spillover, sporadic human cases, and a constant worry about what might happen if it ever gets better at spreading. That kind of quiet escalation is exactly what keeps virologists up at night.
So yes, things are changing. Not as fast as some might like. Not as dramatically as a Hollywood pandemic movie might depict. But slowly, steadily, we are building tools—not just technological, but social, ecological, and procedural—that could change how we live with and eventually prevent the next Nipah outbreak. The virus hasn’t gone away. It hasn’t mutated into something unrecognizable. But we’re getting better at seeing it coming—and, just maybe, at staying one step ahead.
FAQ — Nipah Virus
What is the Nipah virus, and where did it come from?
Nipah virus is a zoonotic pathogen, meaning it jumps from animals to humans. It was first identified in 1998 during an outbreak among pig farmers in Malaysia. The source was traced back to fruit bats—specifically from the Pteropus genus—which are considered the natural reservoir of the virus. Since then, the virus has caused recurrent outbreaks, particularly in South and Southeast Asia, with human cases most often emerging through direct or indirect contact with infected bats, pigs, or even other people.
How does Nipah virus spread from animals to humans?
The most common route is through indirect exposure to materials contaminated by bat saliva or urine. A well-documented example involves date palm sap, a traditional drink in Bangladesh that’s often collected in open containers overnight—just when bats are active. Bats feed on the sap or urinate into the pots, contaminating the liquid. People who drink the raw sap may then become infected. In past outbreaks, pigs also acted as intermediate hosts. And in more recent events, human-to-human transmission—especially among caregivers and healthcare workers—has played an increasingly important role.
Can Nipah virus be transmitted from person to person?
Yes, it can. While earlier outbreaks emphasized animal-to-human transmission, there is now strong evidence of direct person-to-person spread. This typically occurs through close physical contact, exposure to bodily fluids, or aerosolized droplets from coughing. In several outbreaks, transmission chains have formed entirely within families or hospital wards. Fortunately, the virus doesn’t seem to spread as easily as something like measles or COVID-19—but the possibility of it evolving to become more transmissible remains a serious concern.
What are the symptoms of Nipah virus infection?
The symptoms vary widely. Early on, people may just experience fever, fatigue, or headache—very nonspecific. But things can escalate quickly. Severe cases often involve acute encephalitis, where the brain becomes inflamed. This can lead to disorientation, seizures, coma, and death. Some patients also develop respiratory symptoms, ranging from cough and difficulty breathing to full respiratory failure. Even survivors may be left with long-term neurological problems, and in rare cases, the virus can reactivate months later, causing relapsing encephalitis.
How deadly is the Nipah virus?
It’s one of the most lethal viruses known to affect humans. Depending on the outbreak, the case fatality rate has ranged from 40% to as high as 75%. That means up to three out of every four infected people may not survive. Unlike many common viruses, where most people recover, Nipah often overwhelms the body quickly and leaves little room for error.
Is there a treatment or cure for Nipah virus?
There is no specific cure. Supportive care remains the main approach—hydration, respiratory support, and management of complications like seizures. Some experimental treatments are under development. Monoclonal antibodies such as m102.4 have shown potential and were recently granted emergency use in limited settings. Antivirals like ribavirin have been used in past outbreaks, but their effectiveness is unclear. Ultimately, treatment is currently about stabilization, not eradication of the virus.
Is there a vaccine for Nipah virus?
Not yet, but progress is being made. Several vaccine candidates have entered clinical trials. One promising candidate based on a viral vector platform is currently in Phase II testing. The global momentum around vaccine development, bolstered by lessons from COVID-19, is helping to accelerate research. However, a fully licensed, widely available vaccine is still years away.
What can be done to prevent Nipah outbreaks?
Prevention begins with limiting contact between humans and wildlife—especially fruit bats. That includes covering date palm sap containers, managing bat exposure near farms and homes, and reducing habitat encroachment. On the public health side, early detection, rapid isolation, contact tracing, and community education are key. Training healthcare workers and equipping them with proper protective gear is essential to stop nosocomial transmission. Surveillance systems—both ecological and clinical—must be strengthened to catch outbreaks before they spiral.
Is Nipah virus a global threat?
Absolutely, though not in the traditional sense. It doesn’t currently spread around the world the way seasonal flu does, but it has the potential to. All the ingredients are there: a highly lethal virus, the ability to jump species, and documented human-to-human transmission. The fact that it hasn’t caused a pandemic yet is more a reflection of luck and containment than of any inherent limitation in the virus itself. As climate change, deforestation, and global travel patterns continue to evolve, so does the risk.
Conclusion
So where does all of this leave us? Nipah virus is, in many ways, the embodiment of a modern public health dilemma. It’s not everywhere, but it could be. It’s not incurable, but it’s untreatable. We’ve learned enough to fear it, but we haven’t yet done enough to stop it. And that tension—that mixture of knowledge, uncertainty, and vulnerability—is what makes Nipah so important to understand right now.
It challenges us to think differently about disease emergence—not as isolated events, but as symptoms of deeper ecological imbalances. It forces us to confront the limits of our health systems, especially in low-resource settings where outbreaks begin. And perhaps most importantly, it reminds us that waiting for a crisis to escalate before we act is a luxury we can no longer afford.
The future of Nipah research and preparedness doesn’t hinge on one breakthrough. It depends on a web of interventions: scientific, ecological, clinical, social. We need better diagnostics and faster reporting. We need safe caregiving protocols that work in real homes, not just hospitals. We need international coordination that doesn’t stall when headlines fade. And we need communities to be at the center of response efforts—not just as recipients of aid, but as architects of their own resilience.
Nipah virus is not yet a pandemic. But it remains one of the clearest warnings we’ve ever received.
And whether or not it becomes one may depend entirely on how seriously we take that warning—right now.