NanoKnife Pancreatic Cancer Treatment: A Comprehensive Guide
- What Is NanoKnife and How Does It Work in Pancreatic Cancer?
- Types and Stages of Pancreatic Cancer Suitable for NanoKnife
- Comparison of NanoKnife with Traditional Pancreatic Cancer Treatments
- Patient Selection Criteria for NanoKnife Therapy
- Integration of NanoKnife into Multimodal Treatment Plans
- Procedure Overview: What Patients Can Expect During NanoKnife Treatment
- Benefits and Limitations of NanoKnife in Pancreatic Cancer Care
- Clinical Evidence and Survival Outcomes Associated with NanoKnife
- Comparing NanoKnife with Other Localized Therapies
- Role of NanoKnife in Recurrent Pancreatic Cancer
- Patient Selection and Candidacy for NanoKnife Treatment
- Complications and Side Effects: What Patients Should Know
- Current Clinical Trials Involving NanoKnife
- Post-Treatment Monitoring and Imaging Strategies
- Cost Considerations and Insurance Coverage for NanoKnife
- Future Directions in NanoKnife Therapy
- FAQ
What Is NanoKnife and How Does It Work in Pancreatic Cancer?
NanoKnife, also known as irreversible electroporation (IRE), is an innovative, non-thermal ablation technology designed to destroy cancer cells without relying on extreme heat or cold. Unlike traditional radiofrequency or microwave ablation, NanoKnife works by delivering electrical pulses directly into cancerous tissue. These high-voltage pulses create nanopores in the cell membranes, causing irreversible damage that leads to apoptosis, or programmed cell death. The most significant advantage of this technique is its ability to spare nearby vital structures such as blood vessels and bile ducts, which is especially important in pancreatic cancer where tumors often sit close to critical anatomy. Due to this unique characteristic, NanoKnife offers an option for patients with locally advanced pancreatic cancer who are not eligible for surgical resection.
Types and Stages of Pancreatic Cancer Suitable for NanoKnife
NanoKnife is primarily indicated for locally advanced pancreatic cancer (LAPC), which represents tumors that are confined to the pancreas but inoperable due to vascular involvement. It is not typically used for metastatic disease since the therapy is localized and does not target cancer spread throughout the body. For resectable tumors, surgery remains the gold standard, and NanoKnife may be used postoperatively or when microscopic disease is suspected near vital structures. The staging system for pancreatic cancer—based on tumor size (T), nodal involvement (N), and metastasis (M)—helps determine eligibility. Patients categorized as stage III, with no distant metastases but with involvement of critical vessels like the superior mesenteric artery or vein, may benefit most from NanoKnife intervention
Comparison of NanoKnife with Traditional Pancreatic Cancer Treatments
When considering NanoKnife, it is essential to understand how it compares to existing treatments such as chemotherapy, radiation therapy, and surgical resection. Unlike chemotherapy, which works systemically, NanoKnife targets a specific tumor site, reducing the potential for systemic side effects. Radiation therapy, while also localized, may damage nearby organs due to scatter radiation. In contrast, NanoKnife’s non-thermal, targeted approach minimizes collateral damage, making it suitable for delicate areas. Surgical removal of the tumor remains the only potential cure, but not all patients are candidates. In this context, NanoKnife offers a critical therapeutic bridge or an adjunct to improve local control and possibly enhance survival in selected patients. Furthermore, some clinicians are exploring NanoKnife in combination with chemotherapy, hoping to increase the efficacy of both treatments.

Patient Selection Criteria for NanoKnife Therapy
Selecting appropriate candidates for NanoKnife treatment is a multi-disciplinary process involving oncologists, radiologists, and surgeons. The key criterion is a diagnosis of locally advanced pancreatic adenocarcinoma without distant metastasis. Additionally, patients must be in relatively good overall health, as the procedure is typically performed under general anesthesia. Imaging studies such as contrast-enhanced CT or MRI are essential to evaluate the tumor’s proximity to critical structures and to map out electrode placement. Contraindications may include implanted electrical devices like pacemakers, bleeding disorders, or extensive comorbidities. The goal is to identify individuals for whom NanoKnife can offer a survival or quality-of-life benefit without significant procedural risk.
Integration of NanoKnife into Multimodal Treatment Plans
NanoKnife therapy is most effective when incorporated into a comprehensive treatment strategy that may include chemotherapy, radiation, or immunotherapy. For instance, patients often receive induction chemotherapy—such as FOLFIRINOX or gemcitabine-based regimens—prior to NanoKnife to reduce tumor burden and improve outcomes. After tumor size is reduced and stabilized, NanoKnife can precisely ablate residual disease, targeting the area of vascular encasement that typically makes surgical removal impossible. The treatment is also being evaluated as a consolidative option following chemoradiation to achieve maximal local control. This multidisciplinary coordination is essential because treating pancreatic cancer often requires the involvement of surgical oncologists, medical oncologists, radiation oncologists, and interventional radiologists working together in high-volume centers with expertise in advanced ablation technologies.
Procedure Overview: What Patients Can Expect During NanoKnife Treatment
Undergoing NanoKnife therapy involves several pre-procedural and procedural steps. The process begins with detailed imaging—typically CT or MRI—to map out the tumor and surrounding anatomy. Patients are placed under general anesthesia, and interventional radiologists insert multiple needle-like electrodes around the tumor under image guidance. These electrodes deliver a series of high-voltage electrical pulses that cause irreversible damage to cancer cell membranes. Unlike thermal ablation techniques, NanoKnife does not cause coagulative necrosis, making it less likely to harm surrounding structures such as bile ducts or blood vessels. The procedure usually lasts one to two hours, and most patients are monitored overnight in the hospital. Recovery is typically faster than after surgery, and complications are relatively rare when performed by experienced teams.
Benefits and Limitations of NanoKnife in Pancreatic Cancer Care
One of the most compelling benefits of NanoKnife is its non-thermal nature, allowing clinicians to target tumors near critical blood vessels without causing heat-induced damage. This is especially relevant in the pancreas, where tumors often entangle vital arteries and veins. In such cases, traditional surgery is not an option. Another advantage is the minimal recovery time and relatively low rate of post-procedure complications. However, NanoKnife is not curative and is best viewed as a palliative or adjunctive measure. It does not prevent metastasis, nor does it replace systemic therapies needed to control disease spread. Moreover, its availability is limited to specialized centers, and not all insurance plans cover the procedure. Interestingly, researchers studying other metastatic processes such as lung cancer metastasis to breastare beginning to explore similar localized technologies for non-resectable disease.
Clinical Evidence and Survival Outcomes Associated with NanoKnife
The evidence base supporting NanoKnife in pancreatic cancer is growing but still evolving. Several prospective studies and retrospective analyses suggest a potential survival benefit in patients with locally advanced pancreatic cancer treated with IRE following induction chemotherapy. In one frequently cited study, median overall survival reached up to 27 months in selected patients who received NanoKnife after chemotherapy, compared to the historical average of 15–18 months with chemotherapy alone. Additionally, local tumor control rates appear superior, and some patients experience significant pain relief and improved quality of life. Still, randomized controlled trials are limited, and more robust evidence is needed to establish standardized guidelines. As research continues, comparisons to other diagnostics, like whether a DEXA scan can show cancer, highlight the need for both precision imaging and targeted interventions like NanoKnife in comprehensive oncology care.
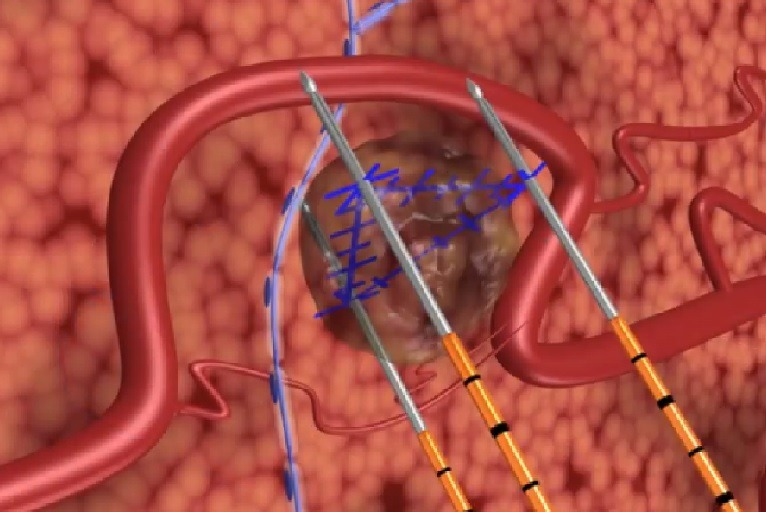
Comparing NanoKnife with Other Localized Therapies
While NanoKnife (IRE) offers unique advantages in pancreatic cancer management, it’s important to compare its efficacy and safety to other local treatment modalities such as radiofrequency ablation (RFA), microwave ablation (MWA), and stereotactic body radiation therapy (SBRT). RFA and MWA use thermal energy, which limits their use near critical structures, whereas NanoKnife’s non-thermal mechanism preserves vital vasculature. SBRT provides a non-invasive option but may not always achieve the same local control in anatomically complex tumors. Additionally, SBRT may require multiple sessions and can lead to radiation-induced side effects in sensitive gastrointestinal tissues. Ultimately, selection of the best modality depends on tumor location, stage, patient performance status, and institutional expertise. Cross-disciplinary tumor boards are instrumental in making these decisions to tailor care to each individual case.
Role of NanoKnife in Recurrent Pancreatic Cancer
Recurrent pancreatic cancer often presents a therapeutic challenge, as previously treated tissues may no longer tolerate further chemotherapy or radiation. NanoKnife has emerged as a valuable tool in this context. Because IRE can target focal recurrences without significantly damaging surrounding tissue, it is increasingly used in patients experiencing isolated local recurrence after initial treatment. These patients may have exhausted surgical and radiation options but still maintain good performance status. The minimally invasive nature of NanoKnife, combined with its precision, makes it suitable for retreatment, potentially extending survival and maintaining quality of life. This approach is being explored in parallel with strategies for managing other difficult cancers, such as early breast cancer skin mets, where recurrence also poses complex treatment decisions.
Patient Selection and Candidacy for NanoKnife Treatment
Not every patient with pancreatic cancer qualifies for NanoKnife. Ideal candidates are those with locally advanced, non-metastatic pancreatic tumors that are deemed unresectable due to vascular involvement. Patients should have a good performance status, acceptable liver and kidney function, and no active infections or coagulopathies. The presence of distant metastasis generally excludes patients from IRE, although clinical trials are beginning to investigate its use in select oligometastatic settings. Prior chemotherapy—typically at least three to four months—is often recommended before NanoKnife to maximize its effectiveness. Institutional protocols may also vary, and patient eligibility is often determined through multidisciplinary review by hepatopancreatobiliary (HPB) teams.
Complications and Side Effects: What Patients Should Know
Although NanoKnife is generally considered safe, it is not without risks. Potential complications include pancreatitis, bleeding, infection, or transient arrhythmias caused by the proximity of electrodes to the heart or diaphragm. However, these events are rare when the procedure is conducted in experienced centers. Most side effects are mild and resolve within a few days post-treatment. Pain at the treatment site and mild fatigue are among the most commonly reported symptoms. It’s also critical for patients to receive preoperative counseling about possible risks and the need for careful postoperative monitoring. As with any cancer intervention, outcomes can vary widely based on tumor characteristics and individual health factors. Emerging comparisons between imaging and ablation, such as how well a PET scan detects colon cancer, also underscore the need for clarity and precision in planning targeted therapies like NanoKnife.
Current Clinical Trials Involving NanoKnife
Clinical trials are expanding the potential applications of NanoKnife, particularly in combination with systemic therapies such as immunotherapy and novel chemotherapeutic regimens. Several Phase I and II trials are exploring how NanoKnife may stimulate the immune system by releasing tumor antigens after ablation, which could synergize with checkpoint inhibitors. Trials are also assessing long-term survival outcomes, tumor progression rates, and quality-of-life metrics compared to conventional treatments. These studies aim to refine patient selection, procedural protocols, and post-treatment care to maximize the benefits of IRE. Participation in clinical trials may be particularly valuable for patients with borderline indications or those who have exhausted standard options. This pursuit of alternative strategies aligns closely with investigations into less conventional approaches for other conditions, such as [alternative cancer treatments for dogs](на русском), where innovation often emerges in response to therapeutic limitations.
Post-Treatment Monitoring and Imaging Strategies
After undergoing NanoKnife treatment, patients require a well-defined monitoring protocol to evaluate treatment response and detect potential recurrence. Contrast-enhanced CT or MRI is typically used 4 to 6 weeks after the procedure to assess changes in tumor size, vascular involvement, and any signs of necrosis. Tumor markers such as CA 19-9 are also monitored regularly. Some centers may incorporate PET scans when there’s uncertainty regarding metabolic activity in the treated area. Continued imaging every three months during the first year is standard practice, with longer intervals thereafter if stability is maintained. Clinicians must differentiate between expected post-ablation changes and signs of disease progression. The use of clear imaging criteria helps avoid misinterpretation, which is especially important when evaluating effectiveness similar to protocols used in DEXA scans and cancer detection.
Cost Considerations and Insurance Coverage for NanoKnife
NanoKnife is a high-precision treatment with significant associated costs. These include hospital fees, anesthesia, equipment usage, and pre- and post-operative imaging. Insurance coverage varies depending on country, insurance provider, and the approval status of NanoKnife for pancreatic cancer in that jurisdiction. In the United States, some private insurers may cover it under compassionate use or investigational device exemptions. Medicare coverage remains limited, generally reserved for clinical trial settings. Financial counselors within cancer centers often assist patients in navigating this process and applying for assistance or grants. The economic feasibility of NanoKnife must be weighed alongside its potential survival benefits, especially in cases where alternative therapies may also carry high costs without the same precision benefits.
Future Directions in NanoKnife Therapy
The future of NanoKnife in pancreatic cancer care is promising and continually evolving. Researchers are working to refine the technology, such as reducing probe size, improving energy delivery control, and integrating real-time image guidance. There is also growing interest in using NanoKnife as a bridge to surgery or as part of a neoadjuvant strategy to downstage tumors. As evidence grows, guidelines may expand its recommended indications, including for other gastrointestinal malignancies. Integration with artificial intelligence in planning electrode placement and treatment zones is also under development. These advances could further increase the safety, efficacy, and accessibility of NanoKnife, offering new hope to patients who otherwise face a grim prognosis.
FAQ
What is NanoKnife and how does it work for pancreatic cancer?
NanoKnife is a non-thermal ablation system that uses electrical pulses to create nanopores in cancer cell membranes, ultimately leading to cell death through a process called irreversible electroporation (IRE). It preserves critical structures like blood vessels and bile ducts, making it especially suitable for treating tumors located near sensitive anatomy in the pancreas.
Is NanoKnife FDA-approved for pancreatic cancer?
While the NanoKnife system has FDA clearance for soft tissue ablation, it is not yet specifically approved for pancreatic cancer. However, it can be used under investigational protocols or within clinical trials for patients who meet certain eligibility criteria, especially those who are not candidates for surgery.
How does NanoKnife compare to traditional surgery?
NanoKnife is not a replacement for surgery but serves as a minimally invasive alternative for patients with inoperable tumors. It offers the benefit of tumor reduction without removing tissue, and it poses fewer risks for complications like bleeding or damage to adjacent vital structures, unlike traditional surgical resections.
What are the eligibility criteria for NanoKnife treatment?
Ideal candidates typically have locally advanced pancreatic cancer that is unresectable but confined without distant metastasis. Tumors must also be accessible by imaging and needle electrode placement, and the patient must be in good overall health to tolerate anesthesia and post-operative recovery.
What are the side effects or risks associated with NanoKnife?
Most patients experience minimal side effects, but possible risks include pancreatitis, infection, bleeding, or temporary pain near the treatment site. Compared to thermal ablation, NanoKnife has a lower risk of collateral tissue damage, which is why it is preferred near major vessels and ducts.
How effective is NanoKnife for pancreatic cancer survival?
Studies suggest NanoKnife can extend survival in select patients, especially those with locally advanced disease. Median survival rates have been reported to increase by several months to over a year, depending on tumor characteristics, treatment timing, and whether additional therapies are used.
Can NanoKnife be combined with chemotherapy or radiation?
Yes, NanoKnife is often used in conjunction with chemotherapy or radiation as part of a multimodal treatment plan. For instance, chemotherapy can be administered before NanoKnife to shrink the tumor, or afterward to control any microscopic disease that remains.
What is the recovery process like after NanoKnife treatment?
Recovery is typically faster than with traditional surgery. Most patients stay in the hospital for 1–3 days and return to normal activities within a week. Post-treatment imaging and blood tests help monitor effectiveness, and supportive care is provided as needed for any symptoms.
Are there any long-term outcomes data available?
Long-term data is still evolving, but early studies show promising results for tumor control and improved survival. Ongoing clinical trials aim to provide stronger evidence, which could eventually lead to standardized protocols and broader use.
How widely available is NanoKnife treatment?
NanoKnife is currently available in select cancer centers and academic hospitals, particularly those with advanced interventional radiology programs. Access may depend on regional medical infrastructure and the availability of trained specialists.
Is NanoKnife painful?
The procedure itself is performed under general anesthesia, so patients do not feel pain during the treatment. Some may experience mild discomfort or soreness afterward, which is generally managed with standard pain relief medication.
What should I expect during the NanoKnife procedure?
Patients are placed under general anesthesia, and image-guided probes are inserted through the skin to the tumor site. A series of controlled electrical pulses are delivered to ablate the tumor. The procedure usually lasts 1–2 hours, followed by short-term observation in a recovery unit.
How is NanoKnife different from radiation therapy?
Radiation relies on ionizing beams to damage cancer DNA, which can also affect surrounding tissues. In contrast, NanoKnife causes direct cellular damage through electric fields without heating tissues, preserving surrounding structures and minimizing radiation-associated complications.
What happens if NanoKnife does not work?
If the tumor does not respond or if there’s recurrence, patients may still pursue other treatments like chemotherapy, radiation, or clinical trial options. NanoKnife does not limit future interventions and can be part of a broader, adaptive cancer care plan.
Can NanoKnife be repeated if cancer comes back?
In some cases, NanoKnife can be repeated depending on tumor location, patient condition, and the nature of the recurrence. However, each situation must be individually evaluated by a multidisciplinary team to determine the safety and potential benefit of repeat treatment.