Medullary Thyroid Cancer and Microcalcifications: A Comprehensive Guide
- Understanding Medullary Thyroid Cancer
- The Significance of Microcalcifications in Thyroid Nodules
- Ultrasound Imaging: Detecting Microcalcifications in MTC
- Fine-Needle Aspiration Biopsy and Cytological Evaluation
- Pathological Features of Medullary Thyroid Cancer
- Surgical Management and Lymph Node Dissection
- Postoperative Surveillance and Imaging
- Prognostic Factors and Risk Stratification
- Genetic Testing and Familial Syndromes
- Treatment Options Beyond Surgery
- Clinical Features and Symptomatology: What Patients Experience
- Biochemical Markers: Calcitonin and CEA in Monitoring
- Pediatric Considerations and Early Detection in High-Risk Families
- Histological Variants and Their Clinical Impact
- Challenges in Diagnosing Small Lesions with Microcalcifications
- What Microcalcifications Reveal in MTC
- Frequently Asked Questions (FAQ)
Understanding Medullary Thyroid Cancer
Medullary thyroid cancer (MTC) is a rare form of thyroid malignancy originating from the parafollicular C cells of the thyroid gland, which are responsible for producing the hormone calcitonin. Unlike the more common papillary and follicular thyroid cancers that arise from follicular cells, MTC accounts for approximately 3% of all thyroid cancer cases. It can occur sporadically or as part of hereditary syndromes, notably Multiple Endocrine Neoplasia type 2 (MEN2). Sporadic cases typically present in adults without a family history, while hereditary forms often manifest earlier and may be associated with other endocrine tumors.
Clinically, MTC may present as a solitary thyroid nodule, often in the upper portion of the thyroid gland. Symptoms can include a palpable neck mass, hoarseness, difficulty swallowing, or diarrhea due to hormone secretion by the tumor. Early detection is crucial, as MTC has a propensity for early lymphatic spread and distant metastases, particularly to the liver, lungs, and bones. Unlike other thyroid cancers, MTC does not absorb radioactive iodine, limiting treatment options and underscoring the importance of surgical intervention.
The Significance of Microcalcifications in Thyroid Nodules
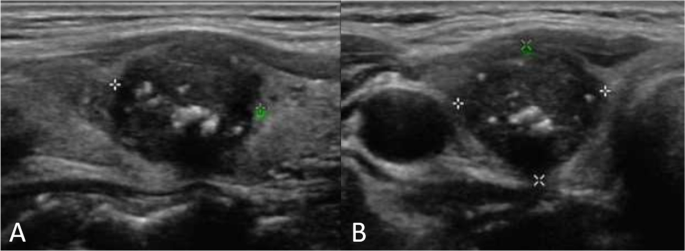
Microcalcifications are tiny calcium deposits that appear as punctate echogenic foci on ultrasound imaging. In the context of thyroid nodules, their presence is considered a suspicious feature, often associated with malignancy. These calcifications are thought to result from psammoma bodies or necrotic debris within the tumor. In medullary thyroid cancer, microcalcifications are observed in approximately 52% of cases, serving as a critical sonographic marker that raises the index of suspicion for malignancy.
The identification of microcalcifications on ultrasound necessitates further evaluation, typically through fine-needle aspiration (FNA) biopsy. Their presence, especially when combined with other suspicious features like hypoechogenicity and irregular margins, significantly increases the likelihood of a malignant diagnosis. Therefore, recognizing microcalcifications is vital in the early detection and management of MTC.
Ultrasound Imaging: Detecting Microcalcifications in MTC
Ultrasound is the primary imaging modality for evaluating thyroid nodules. In medullary thyroid cancer, ultrasound may reveal a hypoechoic nodule with irregular borders, increased vascularity, and the presence of microcalcifications. These microcalcifications appear as tiny, bright echogenic spots without acoustic shadowing, distinguishing them from coarse calcifications seen in benign conditions.
The detection of microcalcifications on ultrasound is significant, as it guides the decision to perform an FNA biopsy. Moreover, ultrasound assists in assessing cervical lymph nodes for metastatic involvement, which is common in MTC. Features such as rounded lymph nodes with loss of the fatty hilum and microcalcifications within the nodes suggest metastatic disease, influencing surgical planning and prognosis.
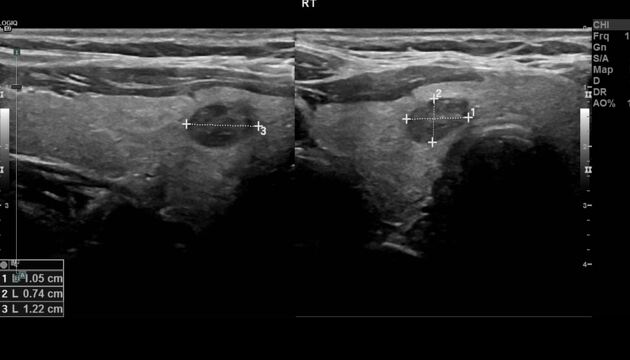
Fine-Needle Aspiration Biopsy and Cytological Evaluation
Fine-needle aspiration (FNA) biopsy is a minimally invasive procedure used to obtain cellular samples from thyroid nodules for cytological analysis. In the evaluation of MTC, FNA plays a pivotal role. However, the cytological diagnosis of MTC can be challenging due to its varied appearance and overlap with other neoplasms. Therefore, adjunctive tests, such as measuring calcitonin levels in the needle washout fluid, are employed to enhance diagnostic accuracy.
The presence of microcalcifications in the aspirated material may support the diagnosis of MTC. Additionally, immunocytochemical staining for calcitonin and carcinoembryonic antigen (CEA) can aid in confirming the diagnosis. Given the potential for false-negative results, especially in small or cystic lesions, a comprehensive approach combining cytology, biochemical markers, and imaging findings is essential for accurate diagnosis.
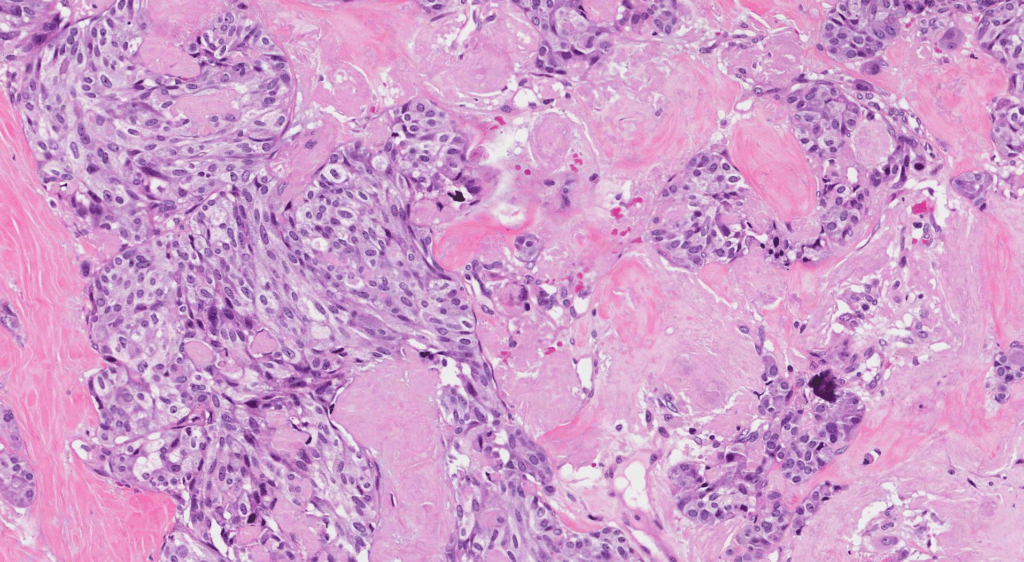
Pathological Features of Medullary Thyroid Cancer
Medullary thyroid cancer displays distinctive histopathological characteristics that set it apart from other thyroid malignancies. Under microscopic examination, MTC typically exhibits polygonal or spindle-shaped tumor cells arranged in nests, sheets, or trabeculae. The presence of amyloid deposits in the stroma is a hallmark finding, composed of altered calcitonin molecules secreted by the neoplastic C cells.
Another significant pathological feature includes the presence of microcalcifications, which may be seen within tumor cell clusters or in association with amyloid-rich areas. These calcifications are not psammoma bodies — as commonly seen in papillary thyroid carcinoma — but rather dystrophic calcifications that form due to necrosis or local degeneration. Identifying these microcalcifications on histology supports the diagnosis and correlates with sonographic findings.
Immunohistochemistry is crucial in confirming the diagnosis of MTC. Tumor cells typically test positive for calcitonin, CEA, chromogranin A, and synaptophysin, confirming their neuroendocrine origin. Genetic testing for RET proto-oncogene mutations is also routinely performed, especially in suspected familial cases. This molecular information influences not only diagnosis but also prognosis and family screening protocols.
Surgical Management and Lymph Node Dissection
Surgery remains the primary and most effective treatment modality for medullary thyroid cancer. Total thyroidectomy is the standard approach, given the multifocal nature of MTC and its tendency to metastasize early. Unlike other thyroid cancers, radioactive iodine therapy is ineffective in MTC due to the non-follicular origin of C cells, making complete surgical excision critical.
In most cases, central compartment (level VI) lymph node dissection is performed prophylactically due to the high likelihood of microscopic metastases, even in clinically negative nodes. If preoperative imaging or intraoperative findings suggest lateral cervical lymph node involvement, a modified radical neck dissection (levels II–V) is warranted.
The presence of microcalcifications in the primary tumor and suspicious lymph nodes on preoperative ultrasound may signal more aggressive disease and prompt a broader surgical field. Experienced endocrine surgeons rely on these imaging cues, in combination with biochemical markers such as calcitonin and CEA, to optimize surgical planning and improve long-term outcomes.
Careful postoperative monitoring is essential, including serial measurements of calcitonin and CEA. Persistent elevation suggests residual disease or early recurrence, prompting further imaging and, potentially, repeat surgery.
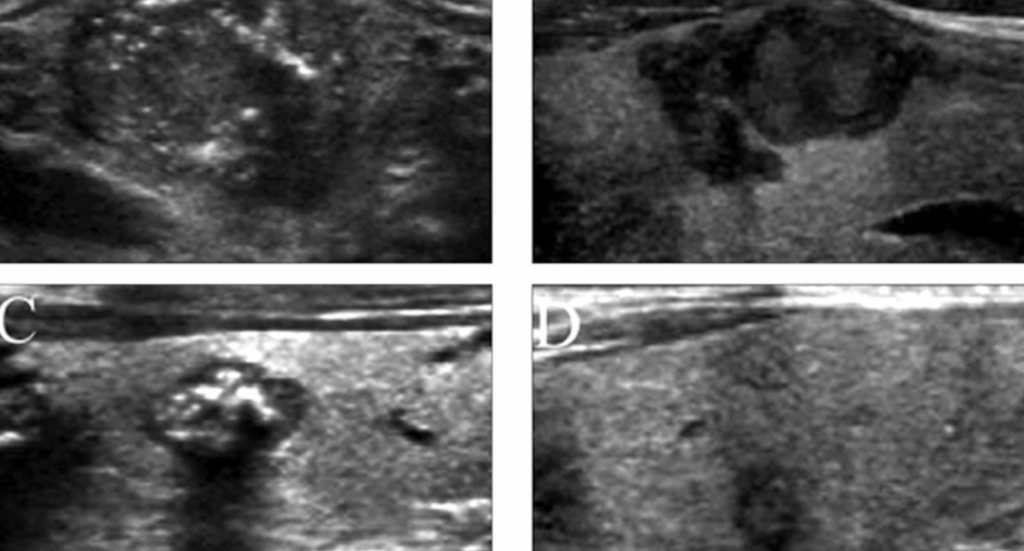
Postoperative Surveillance and Imaging
Long-term follow-up is crucial for patients with medullary thyroid cancer, given its potential for recurrence years after initial treatment. Surveillance involves serial testing of serum calcitonin and carcinoembryonic antigen (CEA) levels, which serve as highly sensitive tumor markers for disease activity.
When either marker is elevated, imaging studies are indicated to localize recurrent or metastatic disease. The most commonly used modalities include:
- Neck ultrasound: to detect residual thyroid tissue, recurrent lymph node metastases, or new nodules
- Contrast-enhanced CT or MRI: especially for mediastinal, thoracic, or liver metastases
- 18F-FDG PET/CT or 68Ga-DOTATATE PET: useful in advanced or biochemically active but radiographically occult disease
Importantly, microcalcifications identified in recurrent nodules or lymph nodes on imaging may signal local recurrence or new foci of malignancy. Their appearance should prompt fine-needle aspiration and cytological evaluation.
In the same way that TULSA-PRO technology enables real-time monitoring during minimally invasive prostate cancer treatment, as discussed in Is tulsa pro the latest procedure for prostate cancer, modern imaging in MTC follow-up plays a similarly dynamic role in guiding intervention strategies.
Prognostic Factors and Risk Stratification
The prognosis for medullary thyroid cancer varies based on a number of clinical, pathological, and molecular factors. Among the most important are:
- Calcitonin doubling time: A rapid increase in calcitonin levels postoperatively is a strong predictor of disease progression and poor survival
- Lymph node involvement: The number and location of metastatic nodes significantly impact recurrence risk
- RET mutation status: Hereditary MTC linked to aggressive RET mutations, such as codon 918 (MEN2B), is associated with early-onset, rapid progression, and worse outcomes
- Tumor size and local invasion: Larger tumors and extrathyroidal extension correlate with reduced disease-free survival
Histopathological features such as the presence of microcalcifications may also reflect a biologically aggressive tumor phenotype. These calcifications, when present in both primary and metastatic sites, have been associated with higher recurrence rates, although more studies are needed to establish this correlation firmly.
As in breast cancer research where dense dose doxorubicin and cyclophosphamide protocols have reshaped treatment in aggressive subtypes, MTC management continues to evolve based on prognostic modeling and molecular profiling.
Genetic Testing and Familial Syndromes
A significant subset of medullary thyroid cancer cases — approximately 25% — arises from germline mutations in the RET proto-oncogene. These hereditary forms are typically associated with Multiple Endocrine Neoplasia type 2 (MEN2) syndromes, which include MEN2A, MEN2B, and familial medullary thyroid carcinoma (FMTC). Genetic testing for RET mutations is critical in all diagnosed patients, regardless of age or family history.
Detection of a RET mutation has implications for both the patient and family members. Individuals with MEN2A may present with MTC, pheochromocytoma, and hyperparathyroidism, while those with MEN2B often exhibit early-onset aggressive MTC, mucosal neuromas, and a marfanoid habitus. Identifying carriers allows for early prophylactic thyroidectomy in children — sometimes as early as infancy — which can be curative if performed before disease onset.
Interestingly, certain RET mutations are associated with increased calcitonin levels and more prominent microcalcifications on imaging, suggesting a link between genotype and sonographic phenotype. As our understanding of RET continues to evolve, personalized surgical timing and surveillance protocols will become increasingly precise.
Treatment Options Beyond Surgery
While surgery remains the cornerstone of therapy for MTC, certain patients — especially those with advanced or recurrent disease — may require additional treatment. These include:
- Targeted therapies: RET inhibitors such as selpercatinib and pralsetinib have demonstrated impressive efficacy in patients with RET-mutant MTC, even in heavily pretreated cases
- External beam radiation therapy (EBRT): used selectively for unresectable tumors or palliation of bone metastases
- Systemic chemotherapy: has limited effectiveness but may be used in aggressive, non-RET-mutant cases
- Peptide receptor radionuclide therapy (PRRT): emerging modality under investigation for somatostatin receptor–positive tumors
The role of these treatments is typically reserved for patients with progressive disease not amenable to surgical resection. Notably, microcalcifications identified in metastatic lesions — such as hepatic or pulmonary nodules — may indicate well-differentiated, slowly progressing disease and can help guide therapeutic decisions.
As seen with TULSA-PRO for prostate cancer, where minimally invasive technology complements systemic therapies, a similar multimodal approach is gaining ground in MTC management — particularly in the metastatic setting.
Clinical Features and Symptomatology: What Patients Experience
Most patients with medullary thyroid cancer present with a painless thyroid nodule. However, due to its neuroendocrine nature, MTC can also produce systemic symptoms related to hormone secretion. The following table outlines key clinical features, their pathophysiology, and diagnostic value:
| Symptom or Sign | Underlying Cause | Clinical Importance |
| Palpable thyroid nodule | Neoplastic growth of C cells | Most common presentation, requires imaging and FNA |
| Diarrhea | Excessive secretion of calcitonin or prostaglandins | Seen in advanced disease, may mimic IBS or infection |
| Hoarseness | Recurrent laryngeal nerve involvement | Suggests local invasion, poorer prognosis |
| Neck fullness/swelling | Lymph node metastasis | Prominent in advanced cases, often with microcalcifications |
| Flushing or facial redness | Hormonal secretion, rare | May indicate aggressive tumor biology |
Some of these symptoms overlap with other systemic conditions. For instance, persistent hoarseness may also be a presenting sign in oropharyngeal tumors — as discussed in the article Difference between vitamin B12 deficiency and tongue cancer — highlighting the importance of considering rare endocrine malignancies in differential diagnosis.
Biochemical Markers: Calcitonin and CEA in Monitoring
Calcitonin is a peptide hormone produced by the parafollicular C cells of the thyroid. In MTC, it serves as a sensitive and specific tumor marker both pre- and postoperatively. Its levels correlate closely with tumor burden, and any elevation after surgery suggests residual or recurrent disease.
Carcinoembryonic antigen (CEA), while less specific, provides complementary information. An increasing trend in either marker — especially a doubling time under 6 months — is associated with a higher risk of disease progression and metastasis. These markers are measured regularly during follow-up, typically every 3–6 months initially, then annually for life.
Interestingly, certain studies have suggested that calcitonin-producing tumors with dense microcalcifications may exhibit slower biochemical progression, making the ultrasound findings potentially useful for prognosis alongside these serum markers.
Together, calcitonin and CEA guide the intensity and frequency of imaging and further treatment — making them indispensable tools in long-term MTC management.
Pediatric Considerations and Early Detection in High-Risk Families
In cases of hereditary MTC, early detection in children is paramount. Carriers of pathogenic RET mutations — especially those with aggressive variants like codon 918 (MEN2B) — are at risk of developing clinically significant medullary thyroid cancer within the first years of life. This necessitates proactive screening strategies in pediatric populations.
Genetic counseling should begin at the time of diagnosis in an index patient. If a mutation is identified, first-degree relatives, including infants, should be tested immediately. Once a child is confirmed to carry the RET mutation, timing of prophylactic thyroidectomy depends on the specific mutation risk level.
For example, children with MEN2B typically require surgery before 1 year of age, while those with less aggressive mutations (e.g., codon 634 in MEN2A) may be monitored with serum calcitonin and imaging until early childhood. High-resolution ultrasound plays a pivotal role in this surveillance, with microcalcifications serving as an early warning sign of transformation from hyperplasia to malignancy.
This proactive approach is similar in spirit to surveillance in other high-risk populations — for instance, young women with familial BRCA mutations undergoing MRI-based screening for breast cancer, or dense dose doxorubicin and cyclophosphamide being considered early in high-risk protocols.
Histological Variants and Their Clinical Impact
Medullary thyroid cancer is not histologically uniform. Several variants exist, and each has implications for prognosis and diagnostic difficulty. The classical variant features a dense cellular pattern with amyloid and calcitonin-positive staining, often accompanied by microcalcifications. However, some rarer subtypes complicate the picture.
- Spindle cell variant: composed of elongated cells, it can be mistaken for sarcoma or anaplastic carcinoma.
- Small cell variant: mimics neuroendocrine carcinoma of the lung and carries a more aggressive course.
- Papillary variant: extremely rare, but may be misclassified as papillary thyroid cancer on cytology, delaying appropriate management.
In some histological subtypes, the presence of microcalcifications is more pronounced and may aid in distinguishing MTC from poorly differentiated neoplasms. Advanced pathology labs now incorporate calcitonin immunostaining and RET mutation sequencing to ensure proper subtype classification.
Understanding these variants is key to choosing the right surgical extent and postoperative strategy — just as tumor biology informs treatment decisions in other cancers such as prostate and colorectal.
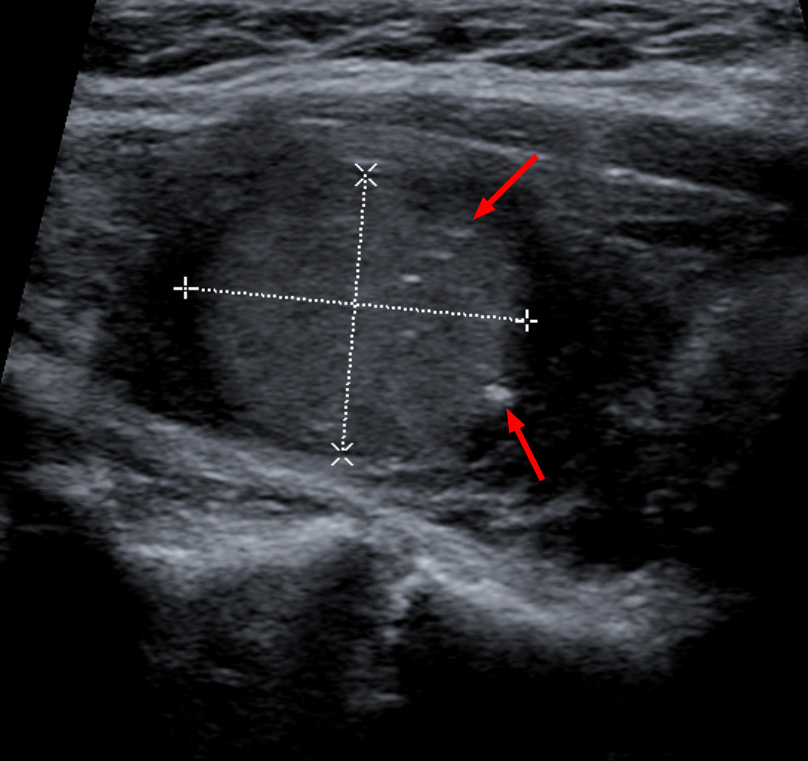
Challenges in Diagnosing Small Lesions with Microcalcifications
One of the most significant challenges in managing MTC is accurately diagnosing small nodules (<1 cm) that exhibit microcalcifications. These lesions may be missed on physical exam and even on conventional ultrasound if the gland is heterogeneous or multinodular.
In such cases, a high degree of clinical suspicion is necessary. A small hypoechoic nodule with even one microcalcification may warrant biopsy, especially in patients with elevated serum calcitonin or a family history. Repeat imaging at 3- to 6-month intervals may be indicated if the initial biopsy is non-diagnostic.
Moreover, cytology alone may not detect early MTC, given its less typical cellular features. Adjuncts like calcitonin washout testing, core needle biopsy, or even early surgical exploration may be necessary for definitive diagnosis.
This mirrors challenges seen in other cancers where minimal lesions — such as low-volume prostate cancers explored in Is tulsa pro the latest procedure for prostate cancer — require advanced imaging or functional biomarkers to avoid delays in care.
What Microcalcifications Reveal in MTC
Microcalcifications in medullary thyroid cancer are not merely an imaging curiosity; they are a window into the tumor’s biology and behavior. Their presence on ultrasound correlates strongly with malignancy and may indicate aggressive features or metastatic potential.
Clinicians must remain vigilant when microcalcifications are observed, especially in high-risk patients or pediatric carriers of RET mutations. These tiny findings often precede clinical symptoms or biomarker changes, allowing for earlier intervention and potentially curative surgery.
As MTC care evolves with molecular diagnostics, advanced imaging, and targeted therapies, the role of microcalcifications remains central — not only in diagnosis but in ongoing surveillance and recurrence monitoring. They exemplify the delicate balance in endocrine oncology between technological precision and clinical intuition.
Frequently Asked Questions (FAQ)
What is medullary thyroid cancer and how is it different from other thyroid cancers?
Medullary thyroid cancer (MTC) is a rare form of thyroid malignancy that arises from the parafollicular C cells, which produce the hormone calcitonin. Unlike papillary and follicular thyroid cancers, MTC does not absorb radioactive iodine and is more often associated with hereditary syndromes, requiring a different diagnostic and treatment approach.
Are microcalcifications always a sign of cancer in thyroid nodules?
Not always, but in the context of thyroid nodules, microcalcifications are considered a highly suspicious feature. They are especially significant when seen in combination with other sonographic features such as irregular margins or hypoechogenicity. In MTC, microcalcifications can be a key early diagnostic clue.
Can microcalcifications be seen in benign thyroid nodules?
Yes, though rarely. Coarse or dystrophic calcifications can occur in benign nodules due to degenerative changes. However, the fine, punctate appearance of microcalcifications on ultrasound is more commonly associated with malignancies, including MTC and papillary thyroid carcinoma.
How are microcalcifications detected in thyroid nodules?
They are most commonly identified via high-resolution ultrasound as tiny bright echoes within a nodule, without acoustic shadowing. This imaging finding often prompts a fine-needle aspiration biopsy to further investigate the nature of the lesion.
Is fine-needle aspiration reliable for diagnosing MTC?
FNA can be helpful but is less sensitive in diagnosing MTC than other thyroid cancers. Supplementary testing, such as calcitonin measurement in the needle washout fluid and immunocytochemistry, increases diagnostic accuracy.
What role does calcitonin play in medullary thyroid cancer?
Calcitonin is both a functional product of the tumor and a sensitive biomarker for MTC. Elevated levels before surgery confirm diagnosis, while postoperative trends are used to detect residual or recurrent disease.
Is surgery always necessary for medullary thyroid cancer?
Yes, total thyroidectomy is the standard treatment for MTC because the cancer does not respond to radioactive iodine. In most cases, lymph node dissection is also performed due to the high rate of early metastasis.
Can medullary thyroid cancer come back after surgery?
Yes, recurrence can occur, especially if the disease was advanced at the time of surgery or if not all affected lymph nodes were removed. Regular monitoring of calcitonin and CEA levels helps detect recurrence early.
What is the prognosis for patients with MTC and microcalcifications?
The prognosis depends on various factors including tumor size, lymph node involvement, and genetic mutations. Microcalcifications may suggest a more biologically active tumor, but do not independently determine prognosis. Early detection and complete surgery significantly improve outcomes.
How often should patients be monitored after treatment?
Monitoring usually involves blood tests for calcitonin and CEA every 3–6 months in the first 2 years, followed by annual checks. Imaging studies are added if tumor markers rise or if new symptoms develop.
Are there targeted therapies available for medullary thyroid cancer?
Yes, RET inhibitors like selpercatinib and pralsetinib are available for patients with RET-mutant MTC. These therapies have shown good response rates in advanced and metastatic cases, especially when surgery is no longer an option.
Can children get medullary thyroid cancer?
Yes, especially in families with inherited RET mutations. In such cases, MTC can develop at a very young age. Early genetic testing and, when necessary, prophylactic thyroidectomy are key to preventing cancer progression.
What imaging tests are used to detect recurrence or metastases?
Neck ultrasound is used for local surveillance. For distant metastases, CT, MRI, or PET/CT (especially 68Ga-DOTATATE) are employed, depending on tumor behavior and marker levels. Microcalcifications in lymph nodes or distant sites on imaging can raise concern for recurrence.
Is medullary thyroid cancer curable?
Yes, especially when diagnosed early and treated with complete surgical resection. In hereditary cases detected through screening, prophylactic surgery can prevent cancer from developing. Even in advanced cases, long-term control is possible with multimodal treatment.
How is medullary thyroid cancer related to other cancers or deficiencies?
Although distinct, MTC can share overlapping symptoms with other conditions, such as hoarseness or neck masses seen in tongue cancer or even in vitamin B12 deficiency. That’s why differential diagnosis — as discussed in related cases like tongue cancer and B12 deficiency — is essential to avoid delays in appropriate care.