TULSA-PRO: The Latest Procedure for Prostate Cancer Treatment
- Understanding TULSA-PRO: An Overview
- The Evolution of Prostate Cancer Treatments
- Detailed Procedure: Step-by-Step Guide
- Benefits and Potential Risks
- Comparing TULSA-PRO with Other Prostate Cancer Treatments
- Patient Selection: Who Is a Candidate for TULSA-PRO?
- Post-Treatment Expectations and Recovery Timeline
- Long-Term Outcomes and Research Evidence
- Comparing Costs and Insurance Coverage
- Role in Salvage and Recurrent Disease
- Potential Complications and How They Are Managed
- Emerging Applications and Future Research
- Is TULSA-PRO the Future of Prostate Cancer Treatment?
- Frequently Asked Questions (FAQ)

Understanding TULSA-PRO: An Overview
TULSA-PRO (Transurethral Ultrasound Ablation) is a non-invasive, image-guided therapy designed specifically for treating prostate cancer and other prostate-related conditions. The acronym reflects its core mechanism: delivering focused ultrasound energy through the urethra under continuous MRI guidance, allowing doctors to precisely target and ablate (destroy) diseased prostate tissue without surgery or radiation.
Unlike conventional treatments like prostatectomy (surgical removal of the prostate), external beam radiation, or brachytherapy, TULSA-PRO is performed without incisions. It uses real-time MRI thermometry to track and control the temperature in the prostate during the procedure. This enables the physician to tailor the therapy to the exact size, shape, and location of the tumor while preserving surrounding healthy structures such as the urethra, neurovascular bundles, and rectal wall.
This innovative method was FDA-cleared in the United States in 2019 and has since been used in clinical centers across North America and Europe. TULSA-PRO is particularly appealing for men with localized low- to intermediate-risk prostate cancer, as well as those with benign prostatic hyperplasia (BPH) or recurrent prostate cancer after prior treatments.
The Evolution of Prostate Cancer Treatments
To understand the significance of TULSA-PRO, it helps to place it in the context of how prostate cancer has traditionally been managed. For decades, the two dominant treatment options were:
- Radical prostatectomy — complete surgical removal of the prostate.
- Radiation therapy — delivered either externally (EBRT) or internally (brachytherapy).
While effective in controlling cancer, both treatments are associated with significant side effects, including urinary incontinence, erectile dysfunction, and rectal damage. Over the years, the medical field has moved toward therapies that provide equal oncological control but with fewer complications.
This led to the emergence of focal therapy — treatments targeting only the cancerous portion of the prostate, preserving as much of the organ and its function as possible. High-Intensity Focused Ultrasound (HIFU) was one of the first in this class, but it lacked the real-time precision and comprehensive imaging that TULSA-PRO now offers.
TULSA-PRO’s combination of MRI mapping and directional ultrasound energy represents the next leap forward. It enables physicians to see, plan, and adjust the treatment in real time — something older technologies simply cannot offer. That’s why it’s being adopted not just as a replacement for older minimally invasive procedures but also as a serious alternative to radical surgery and radiation.
Much like the evolution seen in breast oncology — particularly in the use of dense dose doxorubicin and cyclophosphamide for breast cancer — prostate treatment is shifting toward targeted interventions with fewer long-term side effects.
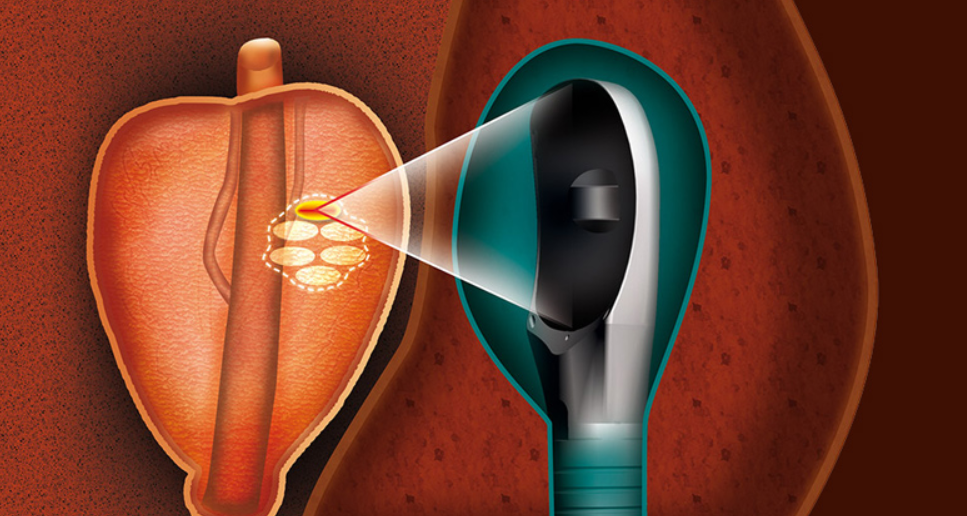
Detailed Procedure: Step-by-Step Guide
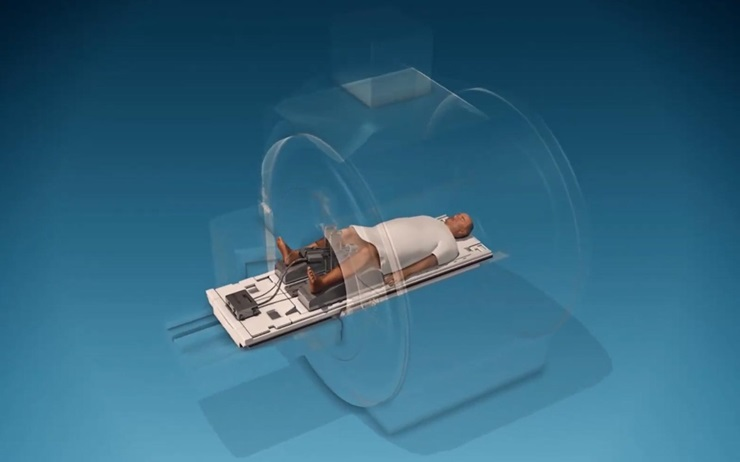
The TULSA-PRO procedure is performed in a hospital or outpatient imaging center under general or spinal anesthesia and typically takes 2 to 4 hours. The following stages highlight the critical steps involved:
Pre-Procedure Planning
Patients first undergo a multiparametric MRI of the prostate to evaluate the size, location, and aggressiveness of the tumor. The physician uses this data to create a precise treatment map, which includes 3D contours of the prostate and adjacent structures to avoid.
Patient Positioning and Device Setup
On the day of the procedure, the patient is positioned inside the MRI suite. A transurethral ultrasound applicator is inserted into the urethra, and a rectal cooling device is simultaneously placed to protect the rectal wall from thermal exposure. Both devices are stabilized, and the MRI imaging is initiated.
Treatment Planning Inside MRI
Using the MRI software, the clinician outlines the areas to be ablated. The software calculates the rotation angles, speed, and intensity of the ultrasound beam to deliver focused energy in a controlled and uniform manner.
Thermal Ablation Execution
The ultrasound transducer rotates and emits directional thermal energy to coagulate prostate tissue layer by layer. The physician monitors tissue temperature in real time via MRI thermometry, making dynamic adjustments to ensure safety and effectiveness. Only the designated cancerous zone is destroyed.
Immediate Post-Ablation Imaging and Recovery
Once the ablation is complete, a second MRI scan verifies that the targeted tissue has been fully treated. The applicators are removed, and the patient is transferred to recovery. Most patients are discharged the same day with only temporary catheterization required.
This level of control and visualization makes TULSA-PRO unique in modern prostate therapy — it’s not just about destroying cancer, but about preserving what matters.
Benefits and Potential Risks
TULSA-PRO offers numerous benefits, particularly in comparison to more aggressive treatments:
Benefits:
- MRI-guided precision reduces harm to nearby organs, improving long-term urinary and sexual function outcomes.
- Minimally invasive: no scalpels, no radiation, no open wounds.
- Faster recovery: patients return to normal activities within days, not weeks.
- Repeatable and adaptable: the procedure can be repeated if necessary or used in combination with other therapies.
- Tailored to anatomy: because of MRI-based customization, physicians can treat different prostate zones with different intensities.
Risks and Considerations:
- Temporary urinary symptoms such as frequency, urgency, or mild incontinence in the weeks following treatment.
- Risk of incomplete ablation in some cases, especially if the cancer is diffuse or located near sensitive structures.
- Erectile function may still be affected, although at much lower rates compared to surgery or radiation.
- Not suitable for men with large-volume high-grade disease, extracapsular extension, or metastasis.
Patients are advised to thoroughly discuss these risks and benefits with a prostate cancer specialist to determine if TULSA-PRO is the right option for their clinical profile.
Comparing TULSA-PRO with Other Prostate Cancer Treatments
To understand the unique value of TULSA-PRO, it’s helpful to directly compare it with other common treatment modalities for localized prostate cancer. This comparison highlights differences in technique, outcomes, and patient experience.
| Aspect | TULSA-PRO | Radical Prostatectomy | External Beam Radiation (EBRT) | High-Intensity Focused Ultrasound (HIFU) |
| Type of Treatment | MRI-guided ultrasound ablation via urethra | Surgical removal of the entire prostate | Radiation delivered from outside the body | Focused ultrasound from rectal probe |
| Invasiveness | Minimally invasive, no incisions | Highly invasive | Non-invasive | Minimally invasive |
| Anesthesia Required | Yes (general or spinal) | Yes (general) | No (outpatient sessions) | Yes |
| Hospital Stay | Outpatient or short stay | 1–3 days | None | Outpatient |
| Imaging Guidance | Real-time MRI | Pre-op imaging only | CT or MRI planning | Ultrasound, less precise |
| Precision | High, with real-time thermal feedback | Moderate (based on surgeon’s experience) | Moderate | Limited compared to MRI-based systems |
| Tissue Sparing | Preserves non-cancerous tissue and critical nerves | Often removes entire prostate and adjacent tissues | May damage surrounding organs | Less tissue sparing than TULSA-PRO |
| Recovery Time | 2–5 days | 3–6 weeks | Several weeks of side effects | 5–10 days |
| Urinary Incontinence Risk | Low (≤5%) | Moderate to high | Moderate | Moderate |
| Erectile Dysfunction Risk | Lower than surgery or EBRT | High | High | Moderate |
| Repeatability | Yes | No | Limited by cumulative dose | Sometimes |
| FDA Approval | Cleared (2019, for prostate tissue ablation) | Fully approved | Fully approved | Cleared for prostate ablation |
| Best For | Localized low to intermediate-risk PCa; salvage therapy; BPH | High-risk or aggressive localized cancers | Older patients; those unsuitable for surgery | Localized PCa, smaller glands |
As shown above, TULSA-PRO stands out due to its ability to balance oncological effectiveness with functional preservation. It is currently considered one of the most promising options for men who prioritize quality of life after cancer treatment.
In some cases, especially when the disease is suspected to be superficial or mistaken for skin manifestations, misdiagnoses can occur — which brings to mind the complexity seen in distinguishing breast cancer: skin mets from dermatological conditions in oncology practice.
Patient Selection: Who Is a Candidate for TULSA-PRO?
TULSA-PRO is not a one-size-fits-all solution. Patient eligibility is based on a number of clinical and anatomical factors that must be evaluated carefully by a urologist or oncologist. The ideal candidate is typically a man with:
- Low to intermediate-risk localized prostate cancer (Gleason score ≤ 7, PSA < 20 ng/mL)
- Benign prostatic hyperplasia (BPH) requiring tissue reduction
- Local recurrence after prior radiotherapy or other focal therapy
Patients with extensive extracapsular extension, lymph node involvement, or distant metastases are not considered good candidates for this procedure. In addition, men with large prostate glands (>80 cc) or prostate calcifications may not respond optimally due to energy delivery limitations.
TULSA-PRO is also being explored in salvage therapy cases — particularly for those who experienced local recurrence after radiation. Its ability to precisely retreat only the affected tissue without significant damage to previously irradiated zones makes it an attractive choice in these complex cases.
A comprehensive evaluation including PSA testing, MRI imaging, and in some cases, biopsy, will determine whether TULSA-PRO is the safest and most effective option. This approach closely mirrors the diagnostic diligence required when distinguishing between symptoms that mimic cancer.
Post-Treatment Expectations and Recovery Timeline
TULSA-PRO’s minimally invasive nature allows for relatively quick recovery. Most patients are discharged on the same day and are able to return to light daily activities within 24–72 hours. However, there are important short-term expectations patients should be aware of:
- A urinary catheter is typically left in place for 3 to 7 days to allow the urinary tract to heal and reduce swelling.
- Urinary symptoms such as urgency, frequency, or a weak stream are common in the first 1–2 weeks but generally improve rapidly.
- Mild pelvic discomfort, perineal soreness, or blood in the urine may occur and is considered normal.
Follow-up includes a post-procedure MRI at 1 to 3 months to assess ablation success and confirm there is no residual tumor. PSA levels are monitored closely, typically showing a significant decline within the first few months.
Unlike radiation, which can have cumulative toxic effects, or surgery with longer recovery time, TULSA-PRO’s gentle tissue handling allows for quicker return to quality of life. Many patients report preserved urinary control and erectile function within weeks — outcomes rarely seen with traditional therapies.
Patients are encouraged to maintain routine follow-ups for PSA tracking and discuss additional imaging if necessary. Importantly, if any symptoms persist, doctors must also consider alternative explanations — including metastatic conditions such as breast cancer: skin mets, which can occasionally present with ambiguous cutaneous changes in advanced stages.
Long-Term Outcomes and Research Evidence
Since its FDA clearance in 2019, TULSA-PRO has been subject to a number of peer-reviewed clinical studies evaluating both oncologic control and functional outcomes. The pivotal TACT trial (TULSA-PRO Ablation Clinical Trial) demonstrated the following:
- Over 75% of men achieved a ≥90% reduction in prostate volume post-treatment.
- 96% of patients with localized cancer showed no evidence of disease progression at 12-month follow-up.
- Urinary incontinence rates remained under 5%, and over 75% of patients retained satisfactory erectile function.
More recently, real-world studies have echoed these results, showing durable cancer control up to 3 years post-procedure. Patients with focal ablation reported especially high quality-of-life scores due to the preservation of healthy prostate tissue and neurovascular integrity.
Research is ongoing to assess TULSA-PRO’s role in high-risk disease, combinational therapies, and use as a salvage procedure. Some studies are also exploring its application in treating BPH without cancer — highlighting its versatility beyond oncology.
As with other evolving treatments in precision medicine — such as dense dose doxorubicin and cyclophosphamide for breast cancer, which transformed chemotherapy protocols — TULSA-PRO reflects a broader shift toward individualized, data-driven cancer care.
Comparing Costs and Insurance Coverage
TULSA-PRO is a specialized procedure typically performed in high-tech medical centers, and its cost can vary significantly depending on the facility, geographic location, and whether it is used for cancer treatment or benign conditions like BPH.
In the United States, the out-of-pocket cost ranges from $15,000 to $25,000, which includes MRI imaging, anesthesia, facility fees, and post-operative monitoring. However, this cost may be partially or fully covered by private insurance when used as a prostate cancer treatment, especially if other options are not feasible or have failed.
Medicare and some private insurers have begun offering partial reimbursement for TULSA-PRO, but it is still not universally covered. Preauthorization is often required, and documentation must demonstrate clinical necessity. When the procedure is used off-label or for retreatment, patients may have to pay entirely out of pocket.
Patients considering TULSA-PRO should consult both their physician and insurer ahead of time. Understanding the financial landscape is just as essential as evaluating clinical outcomes — especially as prostate cancer care increasingly resembles other high-cost, high-tech fields of oncology like targeted breast cancer therapies or dense dose chemotherapy regimens.
Role in Salvage and Recurrent Disease
TULSA-PRO is proving to be particularly useful in salvage therapy for patients who previously received treatment — such as radiation or focal ablation — but experienced recurrence. In these scenarios, re-treatment can be extremely complex due to scar tissue, altered anatomy, and elevated risk of complications.
What sets TULSA-PRO apart is its precision. Since it uses real-time MRI guidance, physicians can define very specific ablation zones and avoid previously treated tissue. Additionally, the transurethral delivery method bypasses rectal access, reducing the risk of complications in patients with prior rectal damage from radiation.
Several salvage-focused studies have shown that TULSA-PRO can achieve excellent local control in recurrent disease with relatively low toxicity. This makes it an ideal option for those who are not candidates for further radiation or surgery. It’s also gaining traction in patients with biochemical recurrence — elevated PSA without visible tumor — where targeted MRI-guided ablation can delay the need for systemic therapies.
In this sense, TULSA-PRO provides an approach comparable in refinement to modern breast cancer salvage regimens, such as re-treatment of skin mets using localized therapies.
Potential Complications and How They Are Managed
While TULSA-PRO has a favorable safety profile, it’s important to understand that no medical intervention is entirely risk-free. The most commonly reported complications include:
- Urinary retention or urgency in the first few days due to swelling of the ablated prostate tissue
- Erectile function changes, typically mild and transient, though permanent effects are possible
- Mild hematuria (blood in urine) and temporary discomfort in the perineal region
- Infection (though rare due to the absence of incisions)
These issues are usually managed conservatively. Catheterization resolves urinary retention, while anti-inflammatory medications help with discomfort. Patients are monitored for any signs of infection or incomplete ablation.
Follow-up imaging is critical to ensure success. In rare cases of incomplete ablation, a second procedure may be scheduled. Because of its flexible targeting system, TULSA-PRO can often be repeated without significant added risk.
This capability to revisit and re-treat lesions reflects a philosophy seen in modern precision oncology — where conditions like tongue cancer or vitamin B12 deficiency are revisited with diagnostic rigor to ensure no hidden pathology is missed.
Emerging Applications and Future Research
Beyond prostate cancer, researchers are exploring other uses for TULSA-PRO, particularly in benign conditions. For example, its ability to precisely ablate tissue with minimal collateral damage makes it attractive for:
- Benign Prostatic Hyperplasia (BPH), especially when medications fail or cause side effects
- Prostate volume reduction prior to brachytherapy
- Non-malignant prostate conditions that still impair urinary flow
Clinical trials are also underway to test the use of TULSA-PRO in combination with immunotherapy or chemotherapy — particularly in advanced-stage or high-risk prostate cancer. There is also interest in its use as part of active surveillance protocols, where patients undergo focal ablation of only the most aggressive lesions while preserving the rest of the gland.
In the next decade, TULSA-PRO may become a modular platform for various prostate interventions — much like how integrated imaging and therapy have evolved in breast and colorectal cancers.
Is TULSA-PRO the Future of Prostate Cancer Treatment?
TULSA-PRO is not just a novel technology — it represents a paradigm shift in how we approach prostate cancer. With MRI-guided precision, real-time temperature monitoring, and non-invasive delivery, it addresses the long-standing tradeoff between cancer control and quality of life.
It offers:
- Real-time adaptability during treatment
- Excellent functional outcomes for urinary and sexual health
- Flexibility for both initial and salvage cases
- Expanding evidence base supporting long-term control
While not suitable for every patient, TULSA-PRO has earned its place among the most promising advances in prostate oncology today. With ongoing trials and broader insurer acceptance, this method is poised to become a standard part of the modern urologist’s toolkit.
Frequently Asked Questions (FAQ)
What is TULSA-PRO and how does it work?
TULSA-PRO is a minimally invasive treatment for prostate cancer and benign prostatic hyperplasia (BPH). It uses directional ultrasound energy, delivered through the urethra, to ablate prostate tissue under real-time MRI guidance. This approach allows physicians to target cancerous areas precisely while preserving nearby healthy structures and minimizing side effects.
Is TULSA-PRO FDA approved for prostate cancer treatment?
Yes, the TULSA-PRO system received FDA clearance in 2019 for the ablation of prostate tissue. While the initial approval included both cancerous and benign indications, its most common clinical use has been in treating localized low- to intermediate-risk prostate cancer.
Who is a good candidate for TULSA-PRO?
Ideal candidates are men with localized, low- or intermediate-risk prostate cancer who want a less invasive alternative to surgery or radiation. It may also be used in cases of BPH or as salvage therapy after prior radiation failure. Eligibility depends on MRI findings, prostate size, cancer location, and overall health.
How is TULSA-PRO different from HIFU or prostatectomy?
Unlike HIFU, which relies on rectal ultrasound and has limited precision, TULSA-PRO uses real-time MRI to guide the ablation with greater accuracy. Compared to radical prostatectomy, TULSA-PRO is non-surgical, sparing more tissue and leading to shorter recovery times with fewer complications.
How long does the procedure take?
The entire process — from setup to completion — typically takes between 2 to 4 hours. The ablation itself lasts 30 to 60 minutes, depending on prostate size and the extent of tissue targeted. Patients usually return home the same day.
Is general anesthesia required?
Yes, TULSA-PRO is performed under general or spinal anesthesia to ensure patient comfort and stillness during MRI-guided targeting. It is not a painful procedure, but precise positioning and immobility are essential for success.
What are the most common side effects?
Short-term side effects include urinary urgency, frequency, mild hematuria (blood in urine), and transient erectile changes. These effects typically resolve within a few weeks. Serious complications are rare, particularly when performed by experienced teams in MRI-equipped centers.
Does TULSA-PRO affect sexual function?
Compared to surgery or radiation, TULSA-PRO has a much lower risk of long-term erectile dysfunction. By preserving the neurovascular bundles that control erections, many patients report recovery of function within weeks. However, outcomes depend on baseline function and treatment extent.
Will I need a catheter after the procedure?
Yes, a urinary catheter is usually placed during the procedure and kept in place for 3 to 7 days post-treatment to allow healing and reduce swelling. Once removed, normal urination typically resumes gradually.
Can the treatment be repeated if necessary?
Yes, one of the advantages of TULSA-PRO is that it can be repeated. If follow-up imaging shows residual disease or recurrence, focal ablation can be performed again with minimal risk, thanks to the high degree of targeting precision.
What kind of follow-up is required?
Patients typically return for MRI imaging 1 to 3 months after the procedure to confirm successful ablation. PSA levels are monitored every 3 to 6 months thereafter. Additional scans or biopsies may be done if there’s suspicion of recurrence.
How soon can I return to normal activities?
Most patients resume light activities within 1 to 3 days. Strenuous exercise, sexual activity, and heavy lifting should be avoided for at least 1–2 weeks. Full recovery is usually achieved within a month, depending on individual response.
Is TULSA-PRO covered by insurance?
Coverage varies by region and insurer. Some private insurers and Medicare plans may offer partial reimbursement for cancer treatment, especially when surgery or radiation are not suitable. Patients should obtain preauthorization and verify with both physician and insurer.
Can TULSA-PRO be used for advanced or high-risk prostate cancer?
Currently, TULSA-PRO is best suited for localized low- to intermediate-risk cases. Its use in high-risk or metastatic disease is limited and typically not recommended. However, trials are underway to evaluate its role as part of combination therapy in more advanced cases.
How does TULSA-PRO compare to other evolving cancer treatments?
TULSA-PRO exemplifies the shift toward image-guided, personalized therapies in oncology — much like MRI-based planning in breast cancer or the use of focused regimens in chemotherapy. It provides a balance between disease control and quality of life, reflecting the goals of modern precision medicine.









