Fibrose Cancer: Understanding the Fibrosis–Cancer Connection
- Foreword
- 1. Introduction to Fibrosis and Cancer
- 2. The Tumor Microenvironment (TME)
- 3. Mechanisms Linking Fibrosis and Cancer
- 4. Organ-Specific Fibrosis and Cancer Associations
- 5. Diagnostic and Prognostic Implications
- 6. Therapeutic Strategies Targeting Fibrosis in Cancer
- 7. Emerging Research and Future Directions
- 8. Patient Perspectives and Quality of Life
- 9. Frequently Asked Questions (FAQ)
- Closing Thoughts
Foreword
Fibrosis and cancer—on the surface, these may seem like separate medical concerns. One conjures images of scarred tissue and chronic inflammation; the other, of rogue cells dividing without restraint. But look closer, and the line between them begins to blur. Fibrosis doesn’t merely coexist with cancer. In many cases, it enables it, shields it, even empowers it. And yet, despite this intimate connection, fibrosis remains an under-discussed player in the cancer story.
This article is meant to change that.
You’re not here for surface-level answers. You’re here because you want to understand what fibrose cancer really is—how fibrosis and malignancy intersect, amplify each other, and shape patient outcomes. Whether you’re a clinician, a researcher, a medical student, or an informed patient or caregiver, this guide aims to be your final stop—a comprehensive, coherent, and clinically grounded resource.
We’ll explore how fibrosis lays the groundwork for cancer development, how tumors manipulate fibrotic pathways for their own survival, and what this means for diagnostics, treatment, and prognosis. We’ll move organ by organ, mechanism by mechanism. We’ll unpack the current science and spotlight where the field is heading next. Along the way, we’ll never lose sight of the lived experience—the person behind the pathology.
And in a world increasingly driven by precision medicine, where success depends on understanding the microenvironment as well as the mutation, this knowledge is not optional. It’s foundational.
So take a deep breath. There’s a lot to cover. But by the end, you’ll see why anyone who wants to understand cancer deeply must also understand fibrosis—and why treating one without acknowledging the other is like fighting a wildfire without noticing the wind.
This article overlaps heavily with tumor microenvironments, and The Eukaryotic Cell Cycle and Cancer offers a complementary look at how cell cycles interact with scarring and chronic damage.
Let’s begin.
Introduction to Fibrosis and Cancer
Let’s start with a question that might seem obvious but is far more layered than it appears: what exactly is fibrosis, and how does it end up tangled in the biology of cancer? These aren’t idle curiosities. They get right to the heart of how diseases progress—not in isolation, but through a web of molecular, cellular, and environmental factors that shape the fate of our tissues.
Fibrosis is essentially your body’s attempt to heal. It’s a wound-healing response that gets dialed up, sometimes catastrophically so. Think of it like a repair crew that never receives the memo to stop building. When tissues are injured—say, by a viral infection, an autoimmune flare, or chronic exposure to toxins—fibroblasts and other repair cells swoop in to lay down extracellular matrix, collagen, and other structural components. Initially, this is protective. But if the damage keeps coming, or if the control systems break down, this response turns pathological. Scar tissue begins to accumulate. Organs stiffen. Cellular communication falters.
So where does cancer fit into this picture?
The connection between fibrosis and cancer isn’t just incidental—it’s intimate. In fact, fibrosis is often a precursor to cancer in organs like the liver, lungs, and pancreas. Chronic injury and inflammation drive fibrosis, which then reshapes the very architecture of tissues. This altered environment doesn’t just tolerate cancer—it invites it. It promotes genetic instability, alters immune surveillance, and creates hypoxic, growth-friendly pockets where mutated cells can thrive.
One of the most striking examples is in the liver. Repeated damage from hepatitis or alcohol abuse leads to fibrosis, which advances to cirrhosis. And cirrhosis, in turn, lays the groundwork for hepatocellular carcinoma. It’s not a one-off pattern; it echoes across different organ systems.
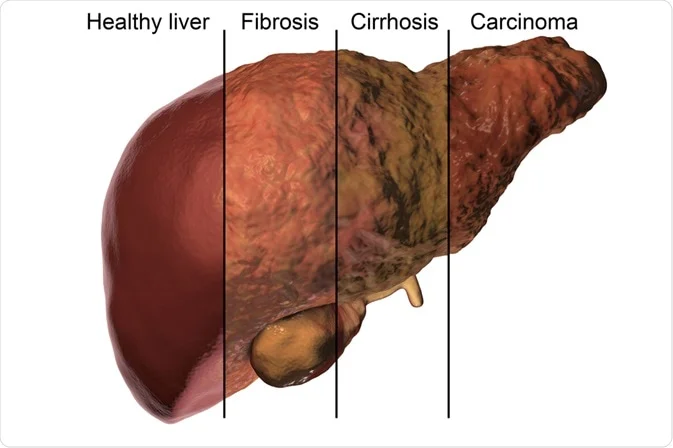
But let’s get precise. If you’re wondering, is fibrosis itself cancerous?, the answer is no—not directly. Fibrosis is not a neoplasm; it doesn’t involve cells breaking free from growth controls in the way malignant tumors do. But it creates a setting—the tumor microenvironment—that is permissive, even supportive, of malignant transformation. The lines between “non-cancerous” and “cancer-prone” can get uncomfortably blurred in fibrotic tissues.
This naturally leads to a few more questions:
- Why do some people with fibrosis develop cancer while others don’t? Genetics, environmental exposures, immune system integrity, and even the microbiome are all being studied as potential modifiers of this risk. The science here is complex and still evolving.
- Is reversing fibrosis enough to prevent cancer? That’s one of the big research questions. Some therapies aim to do just that—by halting fibrotic progression, you may also lower cancer risk. But we’re still learning whether this works consistently in human tissues.
And what about the flip side—can cancer cause fibrosis? Surprisingly, yes. As tumors grow, they often co-opt fibrotic processes. They recruit fibroblasts, stimulate collagen deposition, and essentially build themselves a fibrotic fortress. This not only helps tumors resist treatment (by literally shielding themselves from immune cells and chemotherapy) but also contributes to that stiff, irregular tissue that often characterizes advanced malignancies.
So, to understand cancer fully, we must understand fibrosis—not just as a background condition, but as a co-conspirator.
In the sections that follow, we’ll dissect how this relationship plays out in various organs, explore the molecular underpinnings, and dive into therapies that target both fibrotic and malignant pathways. Because to outsmart cancer, especially the stubborn, aggressive kinds, we must first untangle the scarred and often silent groundwork it thrives upon.
The Tumor Microenvironment (TME)
If you think of cancer as a group of rogue cells going haywire, you’re only getting half the story. Cancer doesn’t develop in a vacuum—it builds its empire within a highly dynamic neighborhood known as the tumor microenvironment, or TME. And like any neighborhood, it shapes—and is shaped by—its residents.
So what is this microenvironment, exactly? It’s the ecosystem surrounding the tumor. It includes everything that’s not the cancer cell itself: blood vessels, immune cells, connective tissue, signaling molecules, and most importantly for our discussion, fibroblasts and the extracellular matrix (ECM). If you imagine a city, the cancer cells are the buildings, but the TME is the infrastructure—the roads, the utilities, the zoning laws, and yes, the corruption that lets things spiral out of control.
Now, let’s get to the heart of the matter: how does fibrosis fit into this environment?
A fibrotic TME is dense, scarred, and biochemically active. Instead of supporting normal function, it fosters chaos. The key player here is the cancer-associated fibroblast (CAF)—a specialized cell that’s been hijacked by the tumor. These cells don’t just passively sit around producing collagen. They secrete growth factors, remodel the ECM, and signal to immune cells in ways that actively support tumor survival and growth. In other words, they’re not innocent bystanders. They’re complicit.
This dense, fibrotic matrix does several things that make life easier for the tumor and harder for everyone else:
- It impedes drug delivery. Think of trying to water a garden through a sponge made of concrete. That’s what chemotherapy faces when trying to reach cancer cells embedded in a fibrotic stroma.
- It alters immune access. The same barriers that block drugs also keep immune cells at bay, making immunotherapies less effective in fibrotic tumors.
- It promotes hypoxia. Dense tissue can restrict blood flow, leading to low-oxygen zones that force cancer cells to adapt and become even more aggressive.
- It enables escape. Fibrosis reshapes the ECM in ways that allow cancer cells to detach, migrate, and invade—essentially opening the door for metastasis.
You might be wondering, is this fibrosis a response to the cancer, or is it helping cause it? The answer is: both. In many cases, fibrosis precedes cancer—particularly in chronic disease settings. But once a tumor takes hold, it begins actively manipulating and worsening the fibrotic environment to its own benefit.
One of the reasons the TME is such a hot area of research is because it represents a therapeutic frontier. Traditional treatments have focused primarily on killing tumor cells. But if the environment is reinforcing the cancer at every turn, doesn’t it make sense to target that too? That’s where anti-fibrotic strategies, TME-modulating drugs, and stroma-targeting immunotherapies come into play.
Of course, this raises another question: can we actually reverse or normalize the TME once it’s gone rogue? Some researchers believe it’s possible. There’s growing interest in “re-educating” fibroblasts, modifying ECM composition, or enhancing vascular access to improve drug delivery. Others advocate for more direct destruction of the fibrotic stroma. The science is still young, but the rationale is strong: if we can reshape the battlefield, the odds of defeating cancer dramatically improve.
One final point before we move on. The tumor microenvironment isn’t static—it changes as the tumor evolves, as treatment is introduced, and as the immune system fights back. It adapts. That’s why understanding fibrosis in cancer isn’t just about studying a static pathology under a microscope. It’s about tracking a living, shifting landscape that plays a starring role in determining who survives, and who doesn’t.
For more real-world scenarios, liver-linked cancers like Stage 4 Neuroendocrine Cancer with Liver Spread often involve fibrotic changes too.
Next, we’ll zoom in even further and unpack the molecular mechanisms linking fibrosis and cancer. If today’s section introduced the city and its corruption, the next will walk you through the blueprints and backroom deals—the specific signaling pathways and cellular behaviors that fuel this alliance.
Mechanisms Linking Fibrosis and Cancer
By now, you’re probably sensing a pattern: fibrosis and cancer aren’t just neighbors—they’re co-conspirators. But the question we need to address now is how this alliance actually works. What are the nuts and bolts? What’s happening at the cellular and molecular level that turns scarring into something far more sinister?
Let’s start with the concept of chronic inflammation, because it’s one of the key initiators of both fibrosis and cancer. Acute inflammation—what you get from a scraped knee or a sore throat—is part of the body’s defense system. It’s supposed to be short-lived, protective, and self-limiting. But when the inflammation persists, as it often does in viral hepatitis, smoking-related lung injury, or autoimmune diseases, it flips a switch. Your immune system doesn’t just repair—it starts remodeling. That means fibroblasts get activated, extracellular matrix accumulates, and tissues slowly harden. Over time, this ongoing wound-healing process creates a microenvironment full of cytokines, growth factors, and reactive oxygen species. And guess what all of those things do? They mutate DNA, interfere with cell-cycle checkpoints, and promote survival signals in cells that should have died.
In other words, chronic inflammation doesn’t just build scar tissue—it builds a launchpad for cancer.
Among the many molecular players in this transformation, Transforming Growth Factor-beta (TGF-β) stands out. TGF-β is like the Dr. Jekyll and Mr. Hyde of cell biology. In healthy tissues, it helps suppress tumors. But in diseased or fibrotic contexts, it shifts roles—promoting fibroblast activation, immune suppression, and even enhancing the invasive potential of cancer cells. It’s as if the cell is re-reading its own instruction manual backwards.
Then there’s Epithelial-Mesenchymal Transition, or EMT—a cellular metamorphosis that deserves more public attention than it gets. EMT is what allows normally stationary epithelial cells to loosen their connections, become motile, and adopt mesenchymal traits. This is crucial in wound healing and embryonic development. But in the fibrotic and cancerous context, EMT is hijacked. It helps epithelial cells break through basement membranes, evade immune detection, and migrate—exactly what you’d want to do if you were a cancer cell aiming to metastasize. It also contributes to fibrogenesis by creating more matrix-producing cells.
Let’s bring another key player into the spotlight: Cancer-Associated Fibroblasts, or CAFs. These aren’t your typical, helpful fibroblasts that knit together your skin after a cut. CAFs are warped by the tumor—they proliferate more, resist cell death, and start pumping out growth factors like FGF, VEGF, and HGF. They remodel the extracellular matrix, create stiffened tissues, and emit signals that protect cancer cells from immune attack. And here’s the kicker: we’re still figuring out where they all come from. Some arise from resident fibroblasts. Others seem to derive from bone marrow stem cells, or even from EMT-transformed epithelial cells. It’s a cellular identity crisis with very real consequences.
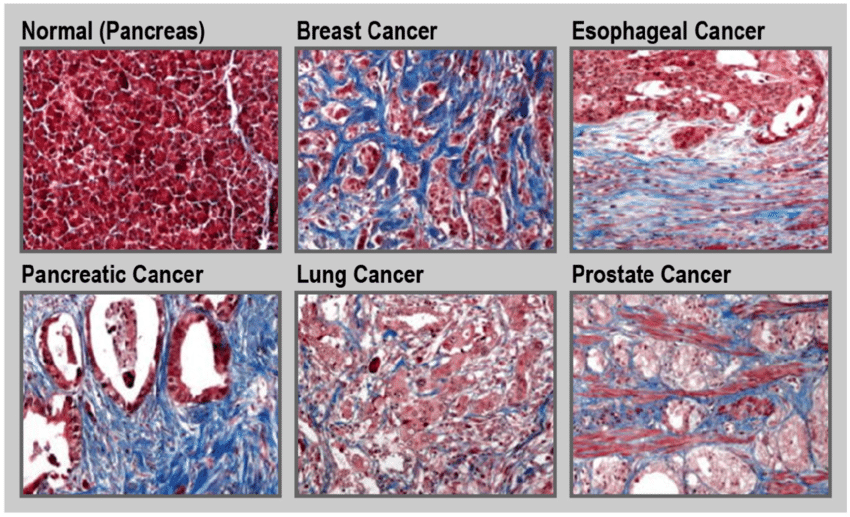
You might now be asking, does every cancer exploit these mechanisms to the same extent? Not quite. Some cancers are far more “fibrosis-heavy” than others. Pancreatic ductal adenocarcinoma, for example, is notoriously desmoplastic—meaning it’s surrounded by dense fibrotic tissue. That fibrosis doesn’t just make the tumor harder to detect and treat—it’s practically part of the tumor’s architecture. In contrast, other cancers like certain leukemias may engage less directly with fibrotic processes.
What ties all of these mechanisms together is the idea that fibrosis and cancer use overlapping strategies for survival, growth, and resistance. And here’s an unsettling truth: therapies that target one pathway often end up influencing the other. For instance, inhibiting TGF-β may reduce fibrosis but could also unmask more aggressive cancer cell behavior if not done carefully. The system is deeply interconnected.
So, what can we take away from all of this?
The biology of fibrosis and cancer is not linear or clean. It’s recursive, self-reinforcing, and context-dependent. In some cases, fibrosis is the first act—laying the groundwork for malignancy. In others, cancer co-opts fibrotic signaling to support its own agenda. Either way, understanding these mechanisms isn’t just academic. It’s the basis for designing smarter, more holistic therapies—ones that recognize that tumors don’t act alone.
In the next section, we’ll shift gears and look at how this relationship plays out in real-world scenarios—organ by organ. Because while the mechanisms may be shared, the way fibrosis and cancer interact in the liver is not the same as in the lungs, the pancreas, or the breast. And that variation may hold the key to truly personalized treatment strategies.
Organ-Specific Fibrosis and Cancer Associations
If you’ve followed the story so far, it’s clear that fibrosis isn’t just a passive background player in cancer biology—it’s often a strategic enabler. But this relationship isn’t uniform across the body. Different organs, different tissues, and different disease contexts can radically change how fibrosis and cancer interact. To really understand this duo, we need to zoom in and get granular.
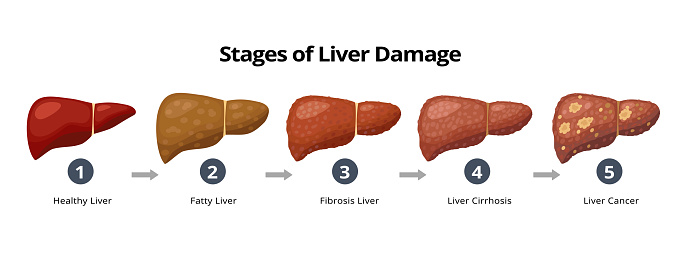
Let’s begin where the connection is perhaps most established: the liver.
Chronic liver injury—be it from hepatitis B or C, long-term alcohol use, or increasingly, nonalcoholic fatty liver disease (NAFLD)—leads to fibrosis, and then cirrhosis. The process is slow, often unfolding over years or decades. But here’s the key: once cirrhosis sets in, the risk of hepatocellular carcinoma (HCC) spikes dramatically. Why? Because cirrhosis is not just scar tissue. It’s a distorted microenvironment filled with inflammatory cells, fibrogenic signals, and areas of cell death and regeneration. This chaotic regenerative state is fertile ground for malignant transformation. In fact, in many regions of the world, HCC is one of the most common cancers—and fibrosis is nearly always part of the prelude.
Next up: the lungs. Pulmonary fibrosis, especially idiopathic pulmonary fibrosis (IPF), has a well-documented link to lung cancer—particularly non-small cell lung cancer (NSCLC). This might seem counterintuitive at first. After all, fibrosis stiffens the lung tissue, making it less hospitable, right? But the stiffening is the problem. It disrupts normal tissue architecture, creates chronic hypoxia, and keeps the immune system in a constant low-grade activation state. Over time, that remodeling lays down molecular landmines—mutations, epigenetic changes, and dysregulated repair mechanisms—that prime the lung for cancer. Patients with IPF have up to a fivefold increased risk of developing lung cancer, and the prognosis tends to be worse due to delayed detection and limited treatment options.
The pancreas tells an even more aggressive story. Pancreatic ductal adenocarcinoma (PDAC) is infamous not just for its lethality but for its intensely fibrotic stroma—called desmoplasia. Here, fibrosis isn’t just part of the picture. It is the landscape. CAFs, immune cells, and rigid ECM dominate the tumor microenvironment. This dense matrix physically compresses blood vessels, reducing drug delivery, while biochemically nurturing tumor cells with growth factors and protective signals. You might ask, does the fibrosis cause the cancer, or does the cancer cause the fibrosis? In PDAC, it’s both. Chronic pancreatitis—a known risk factor—can lead to pancreatic fibrosis and eventually cancer. Once the tumor arises, it doubles down on the fibrosis, feeding off its own scar tissue.
Breast tissue offers a more nuanced case. The breast has a naturally complex stromal architecture, and the role of fibrosis here is under intense investigation. What we do know is that fibrotic, collagen-dense breast tissue is not benign. In fact, high mammographic density—a proxy for stromal and fibrotic content—is one of the strongest independent risk factors for breast cancer. Why? Because the ECM composition alters cellular polarity, growth signals, and immune cell behavior. Stiff tissue isn’t just harder for radiologists to interpret—it’s an active participant in tumorigenesis. And in established tumors, CAFs once again show up to shape the battlefield, contributing to recurrence and resistance.
There are other organs where fibrosis-cancer links are emerging but not yet fully mapped. For example:
- In the kidneys, chronic injury and scarring are associated with higher rates of renal cell carcinoma, especially in transplant recipients and patients with polycystic kidney disease.
- In the prostate, certain forms of stromal remodeling seem to precede aggressive cancer behavior, though the causality is less clear.
- In the esophagus, chronic acid reflux can lead to Barrett’s esophagus, where inflammation and fibrosis pave the way for esophageal adenocarcinoma.
It’s worth emphasizing that fibrosis isn’t just “bystander biology” here. It’s often predictive. In many of these organs, fibrosis serves as an early warning system—one that clinicians and researchers are learning to read more carefully. Fibrosis can signal elevated cancer risk before any tumor is visible. That’s why imaging techniques, biomarker development, and even artificial intelligence systems are now being trained to detect and interpret fibrotic signatures.
So, what should we take from this tour through the body?
First, the fibrosis-cancer link is not anecdotal. It is anatomically specific, mechanistically rich, and clinically relevant. Second, while fibrosis might manifest differently in each organ, the underlying themes—chronic damage, immune modulation, ECM remodeling—are consistent. Third, and perhaps most importantly, fibrosis is increasingly seen as a modifiable risk factor. That changes the game. If we can detect and intervene in fibrotic pathways early, we may not just delay cancer—we may prevent it entirely.
Up next: we’ll turn to how fibrosis and cancer are identified and monitored in the clinic. Because understanding this link isn’t just academic. It has profound diagnostic and prognostic implications—and we’re only beginning to tap into that potential.
Diagnostic and Prognostic Implications
So far, we’ve examined how fibrosis isn’t just a companion to cancer—it’s often a signal, a scaffolding, even an accomplice. That naturally leads to the next question: how do we detect fibrosis when it matters, and what can it tell us about cancer risk, progression, or treatment outcomes?
The answer, as with much in oncology, is both promising and frustrating. Fibrosis can be surprisingly elusive. It doesn’t announce itself with the clarity of a tumor mass or a rogue biomarker. It’s woven into the background of tissues—often silently, gradually—until one day it starts changing the rules of cellular behavior. But that doesn’t mean we’re helpless. Let’s walk through how clinicians and researchers are detecting fibrosis and leveraging that information in the fight against cancer.
First, there’s imaging, which remains the frontline tool for spotting structural changes in tissues. Techniques like CT scans, MRI, and PET scans can sometimes pick up dense, fibrotic regions—especially in organs like the liver and lungs. In liver disease, for example, elastography (a specialized form of ultrasound) can measure tissue stiffness, which correlates with the degree of fibrosis. That’s not just helpful for staging liver disease—it’s become a proxy marker for hepatocellular carcinoma risk. In the lungs, high-resolution CT can reveal honeycombing and other architectural distortions characteristic of pulmonary fibrosis—signals that might nudge a clinician to screen more aggressively for lung cancer in high-risk patients.
But imaging, useful as it is, often lacks nuance. It tells you that something is there—but not always what it’s doing. That’s where biomarkers come in.
Researchers are constantly hunting for molecular signatures of fibrosis—proteins, enzymes, RNA fragments—that might show up in blood, urine, or tissue samples. Some of the current candidates include:
- Procollagen peptides (like PIIINP), which reflect active collagen synthesis.
- TGF-β levels, which, as we’ve discussed, play a dual role in fibrosis and tumor behavior.
- Fibroblast activation protein (FAP), which is expressed by cancer-associated fibroblasts and may also be targetable.
- Galectin-3 and other signaling molecules involved in fibrosis-driven inflammation.
Now, are these biomarkers ready for prime time? Some are used in niche clinical settings, while others remain in the research phase. But the goal is clear: develop noninvasive or minimally invasive tests that can detect fibrosis early, quantify its activity, and stratify patients based on risk—not just for fibrosis progression, but for cancer development or recurrence.
That brings us to the histopathological evaluation, the gold standard. When doctors biopsy a suspicious lesion—whether in the liver, pancreas, breast, or elsewhere—they often see fibrotic features under the microscope. These include collagen deposits, activated fibroblasts, and changes in tissue architecture. What’s fascinating is that the degree and pattern of fibrosis can provide prognostic clues. A tumor with a dense, desmoplastic stroma might be more aggressive, more resistant to therapy, or more likely to recur. Pathologists have learned to “read” these fibrotic cues as part of the tumor’s behavior profile.
This is where prognosis comes in—and where fibrosis becomes more than just a descriptive feature. In many cancers, the presence and extent of fibrosis correlate with worse outcomes. Not always, and not universally—but often enough to matter.
- In pancreatic cancer, high stromal density is associated with poorer survival.
- In breast cancer, a stiffer ECM environment has been linked to greater metastatic potential.
- In liver cancer, underlying cirrhosis complicates treatment and impacts long-term survival, even after resection.
So yes, fibrosis can be a predictor—not just of cancer risk, but of how a known cancer might behave.
One of the newer frontiers here is radiomics, where imaging data is processed using AI to extract features that humans might miss. These algorithms can detect subtle textural patterns—some of which correspond to fibrosis—and link them to outcomes in ways that are only now being validated. It’s not science fiction. It’s the next step toward predictive pathology powered by digital tools.
You might ask: could we get to a point where fibrosis alone triggers a preemptive response? In some settings, we already do. Patients with advanced liver fibrosis (but no cancer yet) are often put on regular HCC surveillance. Some researchers are exploring whether fibrotic patterns on breast imaging could become part of a more nuanced risk model, beyond just family history and genetic markers.
But we’re still in early days. The challenge is that fibrosis is complex—it can be active or static, diffuse or localized, benign or foreboding. The context matters. The organ matters. The coexisting biology matters.
Still, the overarching message is this: fibrosis is measurable, interpretable, and increasingly actionable. It’s a diagnostic and prognostic clue that we’re learning to decode—and that could soon shape screening strategies, treatment decisions, and even drug development.
Next, we’ll look at what’s being done on the therapeutic front. Because if fibrosis is more than just a symptom—if it’s part of the problem—then the next logical question is: can we treat it, and in doing so, treat cancer more effectively too?
Therapeutic Strategies Targeting Fibrosis in Cancer
Let’s take a moment to recap where we are. We’ve established that fibrosis is not just a side effect of disease but an active player—sometimes a lead actor—in the story of cancer. It shapes the tumor microenvironment, predicts clinical outcomes, and interferes with treatment. That naturally raises the question: can we target fibrosis therapeutically? And if so, can we improve cancer outcomes in the process?
The answer, as with many things in medicine, is “yes—but.” There is immense therapeutic potential here, but it’s complicated. Fibrosis, after all, is a process driven by many of the same mechanisms that support normal wound healing. Target it too aggressively, and you risk impairing tissue repair or triggering off-target effects. But done thoughtfully—strategically—we might just be able to alter the course of some of the most stubborn cancers.
Let’s start with the most intuitive approach: anti-fibrotic agents. Some of these were originally developed for fibrotic diseases like idiopathic pulmonary fibrosis or cirrhosis but are now being explored in oncology. Take pirfenidone and nintedanib, for example—both are approved for IPF and have shown potential to reduce fibroblast activation and extracellular matrix deposition. Early studies are investigating whether they could modulate the fibrotic stroma in tumors, especially in fibrosis-heavy cancers like pancreatic or liver cancer.
Then there are TGF-β inhibitors, which target one of the central signaling pathways in both fibrosis and cancer. You might recall from earlier sections that TGF-β plays a double role—tumor suppressor in early disease, tumor promoter in later stages. This duality makes it a tricky target. But in late-stage cancers with dense fibrotic microenvironments, blocking TGF-β may reduce CAF activation, loosen the ECM, and improve immune infiltration. Clinical trials are underway to see if combining TGF-β inhibitors with checkpoint inhibitors (like PD-1 or CTLA-4 blockers) can unmask “cold” tumors and make them responsive to immunotherapy.
Which brings us to a broader point: fibrosis can be immunosuppressive. When the tumor stroma gets too dense, it acts like a biochemical moat—keeping T cells and other immune cells out. This is a major reason why some tumors, particularly pancreatic and some colorectal cancers, respond poorly to immunotherapy. So researchers are experimenting with stroma-modulating agents—drugs that don’t kill the tumor directly but remodel the surrounding environment. These include hyaluronidase enzymes (like PEGPH20) that break down components of the ECM, theoretically improving drug delivery and immune cell access. The results so far? Mixed. Some trials have shown promise, others have not. But the concept is solid, and the next generation of stroma-modulating drugs is smarter, more selective, and potentially more synergistic with existing therapies.
One especially interesting area is targeting cancer-associated fibroblasts (CAFs). Unlike normal fibroblasts, CAFs are persistently activated and often help the tumor evade therapy. But they’re not uniform—there are subsets of CAFs, some more pro-tumorigenic than others. This has led to an effort to classify CAF populations and selectively target the bad actors. One experimental approach involves inhibiting fibroblast activation protein (FAP)—a surface marker expressed by many CAFs. FAP-targeted therapies, including CAR-T cells and antibody-drug conjugates, are currently in early-phase trials.
Another emerging idea is reprogramming rather than destroying the fibroblasts. Instead of trying to kill all CAFs (which may cause more harm than good), what if we could coax them back to a more quiescent, “normal” state? Some epigenetic drugs and small molecules are being developed to do just that—to alter fibroblast behavior without wholesale elimination. It’s a gentler, potentially more sustainable approach.
Now, if you’re thinking this is all very promising but still early-stage, you’re right. Many of these strategies are in clinical trials, not yet in standard oncology toolkits. But they represent a shift in thinking. Instead of focusing only on the tumor, we’re recognizing that the environment matters—and that fibrosis isn’t just a marker of aggressiveness, but a targetable feature in its own right.
And let’s not forget about combination therapy, which may ultimately be the most powerful strategy. Monotherapies rarely work in advanced cancer. But combining anti-fibrotic agents with chemotherapy, immunotherapy, or radiation may allow each component to work more effectively. In animal models of pancreatic cancer, breaking down the fibrotic stroma has made previously unresponsive tumors suddenly vulnerable to T cell attack. Clinical trials are now trying to replicate that in humans.
Of course, we need to be careful. Not all fibrosis is bad. In some contexts, a fibrotic capsule around a tumor may actually limit its spread. So we have to be selective—targeting the right type of fibrosis, in the right patients, at the right stage of disease. That’s where biomarkers, imaging, and personalized medicine come into play—tools that can help us figure out when fibrosis is a foe and when it’s merely a bystander.
Also worth considering is how systemic terrain models fit in, which we unpack in The Metabolic Approach to Cancer.
All of this points to a broader evolution in oncology: one that sees the tumor as part of a complex tissue ecosystem. By targeting that ecosystem—and the fibrotic scaffolding that supports it—we may finally begin to chip away at cancers that have, until now, resisted everything we’ve thrown at them.
Emerging Research and Future Directions
We’ve covered a lot of ground—from the foundational biology of fibrosis and its role in cancer, to diagnostics, and the latest therapeutic strategies. But the truth is, we’re only scratching the surface. As science often does, the more we uncover about the fibrosis-cancer axis, the more questions we raise. What’s exciting now is not just where we are, but where we’re headed. The field is evolving rapidly, and the next wave of research is poised to reshape how we understand and treat these diseases—possibly even how we prevent them.
Let’s begin with a deceptively simple question: can fibrosis be intercepted before it drives cancer? That’s the ultimate goal of several ongoing studies. The idea is to shift from reactive treatment to proactive prevention—identifying people at high risk based on fibrotic signatures, and intervening before malignancy takes hold. Sounds ambitious? It is. But with advances in molecular profiling, AI-assisted imaging, and liquid biopsies, we’re inching closer to making this real.
One major frontier lies in genetic and epigenetic research. We now know that certain individuals are genetically predisposed to more aggressive fibrotic responses. Variants in genes regulating collagen production, TGF-β signaling, and immune modulation can all influence whether a person’s chronic inflammation turns into fibrosis—and whether that fibrosis turns malignant. What’s more, epigenetic changes (those heritable alterations that don’t affect the DNA sequence itself but do change gene expression) are increasingly recognized as key drivers of both fibrotic activation and cancer evolution. Targeting these epigenetic regulators may allow us to reverse fibrotic programming in cells before it spirals out of control.
Closely related to this is the growing field of precision oncology, which seeks to tailor treatments not just to the tumor’s genetic makeup, but to the stromal and fibrotic landscape of the tumor microenvironment. Imagine a world where we don’t just sequence a tumor’s DNA, but also map its fibrotic density, immune infiltration, and fibroblast composition. We’re not quite there yet, but multi-omic profiling platforms—those that combine genomics, transcriptomics, proteomics, and spatial analysis—are making that a plausible future.
Then there’s the wild card: biomaterials and tissue engineering. Researchers are creating 3D models of fibrotic tumors using synthetic scaffolds and organoids—miniature, lab-grown versions of human organs. Why does this matter? Because it allows us to study how fibrosis physically and biochemically alters tumor behavior in ways that animal models can’t replicate. These systems are already being used to test anti-fibrotic and anti-cancer drugs in tandem, speeding up the preclinical research pipeline and providing insights that could translate more directly into patient care.
You may be wondering, what about the immune system? Doesn’t it play a huge role here? Absolutely. And that’s why the integration of immunology and fibrosis research is a top priority. We’ve already discussed how fibrosis can block immune access and promote immune suppression, but researchers are now trying to flip that dynamic. Some are exploring immune checkpoint inhibitors that also target fibrotic mediators. Others are developing vaccines against fibrotic epitopes, or training CAR-T cells to recognize FAP-expressing fibroblasts. If this sounds like science fiction, give it a year or two—it may soon be science fact.
Another fascinating angle is the microbiome—the trillions of microorganisms living in and on our bodies. It turns out the gut microbiome can influence systemic inflammation and fibrosis, particularly in the liver and pancreas. Some bacteria may promote fibrotic signaling through metabolites and immune interactions; others may have protective effects. Modulating the microbiome—through diet, probiotics, or even fecal transplants—is being explored as a way to reduce fibrosis and, by extension, lower cancer risk.
Finally, let’s talk about preventive strategies. If fibrosis is a modifiable risk factor, shouldn’t we be doing more to prevent it in the first place? Absolutely. In liver disease, interventions like antiviral therapy for hepatitis, lifestyle changes to reduce fatty liver disease, or antifibrotic drugs in early fibrosis are already part of the conversation. But what if we could extend that logic to the lungs, the pancreas, the breast? Preventive oncology doesn’t have to start with a tumor. It can start with the microenvironment.
All of this raises a final, critical point: fibrosis may be the earliest biomarker of cancer risk that we’re just now learning how to read. Long before a mass shows up on a scan or a biopsy yields a malignant result, fibrosis may be whispering that something is going wrong. The challenge is listening closely enough, early enough, and accurately enough to respond.
We’re not there yet. But the research is moving fast, and the future is full of possibilities. In the next section, we’ll step back from the science and look at what all of this means for patients—not in theory, but in day-to-day life. Because fibrosis isn’t just a molecular phenomenon. It’s something people live with, struggle with, and endure—often alongside cancer itself.
Patient Perspectives and Quality of Life
We’ve now waded deep into molecular pathways, therapeutic strategies, and research frontiers—but if we stop there, we’re missing something essential. At the center of all this science is a very human story. Because while fibrosis and cancer can be modeled in labs and dissected in journals, their true impact is felt most vividly in the bodies, minds, and lives of people who have to live with them—often simultaneously. And this experience is far more than a collection of symptoms. It’s a chronic negotiation with discomfort, uncertainty, and adaptation.
So what does it feel like to live with fibrosis and cancer? It depends on the organ involved, the stage of disease, and the person’s overall health. But one common thread is that fibrosis often complicates everything.
For instance, patients with cirrhosis and liver cancer may feel fatigued not just from the tumor burden, but from the systemic effects of fibrotic liver dysfunction—muscle wasting, poor nutrient absorption, clotting abnormalities. Those with idiopathic pulmonary fibrosis and lung cancer may find their respiratory symptoms blur together—persistent cough, shortness of breath, exercise intolerance—all of which can quietly erode quality of life long before a cancer diagnosis is even made.
And that’s a major issue: fibrosis often masks or mimics cancer symptoms, which delays diagnosis. Some patients are told they have “just” fibrosis—until one day the scans tell a different story. Others receive a cancer diagnosis only to find their treatment options limited because their underlying fibrotic disease makes surgery too risky, or chemotherapy too toxic.
Here’s where the emotional and psychological load starts to weigh in. It’s one thing to confront a cancer diagnosis. It’s another to learn that your body is not just fighting a tumor, but also remodeling itself against you in slow, irreversible ways. The sense of betrayal—of being trapped in a body that’s healing the wrong way—can be deeply unsettling. Add to that the fact that many fibrotic conditions are chronic and progressive, with few clear treatment options, and you’ve got a recipe for long-term emotional strain.
You might ask: do patients with both fibrosis and cancer have worse outcomes? Statistically, often yes. But that doesn’t mean the outlook is hopeless. It means clinicians must take a whole-body, whole-person approach—not just treating the tumor, but managing the fibrosis and its fallout at every step.
That means addressing:
- Symptom management. Pain, fatigue, breathlessness, and gastrointestinal issues are common and often overlapping. Effective palliation and supportive care aren’t luxuries here—they’re fundamental.
- Treatment customization. Many standard cancer therapies are harder to deliver in fibrotic patients. The liver, for example, processes most chemotherapy agents. If it’s already compromised by cirrhosis, doses may need to be adjusted—or entirely new regimens considered. Similarly, lung fibrosis limits tolerance for radiation or surgery.
- Mental health and psychosocial support. Chronic disease plus cancer is a psychological double burden. Depression, anxiety, and isolation are common, especially when patients feel that no one really understands the full complexity of their condition.
- Communication and coordination. Patients with both fibrosis and cancer often have to navigate multiple specialists: oncologists, pulmonologists, hepatologists, rheumatologists. When communication breaks down—or when care becomes fragmented—patients suffer. Integrated care models, where specialists work as a team, aren’t just ideal. They’re necessary.
And then there’s the question of hope. How do we talk about prognosis in this context? Carefully. With nuance. The truth is, having fibrosis doesn’t mean cancer treatment is futile. But it does mean that risks and benefits must be weighed with even greater precision. It means involving patients in decision-making, respecting their values and goals, and being honest about what we know—and what we can’t predict.
Interestingly, some patients find that addressing fibrosis gives them a clearer sense of agency than cancer treatment alone. Anti-fibrotic medications, lifestyle changes, nutrition, even physical therapy can all play a role in slowing fibrotic progression and improving daily function. In this sense, managing fibrosis isn’t just adjunct to cancer care—it’s part of it. And it can be empowering.
Caregivers, too, play a vital role—and often a heavy one. Supporting someone with dual diagnoses can be emotionally and physically exhausting. Caregivers need support systems of their own: respite, counseling, education. And they need to be part of the conversation, not just the logistics.
So what’s the takeaway?
The experience of living with fibrosis and cancer is complex, often frustrating, but not without resilience or progress. Patients aren’t just navigating biology—they’re navigating systems, emotions, relationships, and futures. And any scientific advance that fails to recognize that—no matter how technically brilliant—is incomplete.
Frequently Asked Questions (FAQ)
1. What is the relationship between fibrosis and cancer?
This is the foundational question, and the answer isn’t simple—but it is increasingly clear. Fibrosis, especially when it’s chronic and active, creates an environment that fosters the development and progression of cancer. Through persistent inflammation, tissue remodeling, and changes in cellular signaling, fibrosis can turn once-protective processes into cancer-friendly terrain. In many cases, fibrosis doesn’t merely coexist with cancer—it helps shape the conditions under which it arises and thrives.
2. Can fibrosis be a precursor to cancer?
Yes, and in many organ systems, it often is. Liver fibrosis progressing to cirrhosis is a prime example, where the fibrotic process significantly increases the risk of hepatocellular carcinoma. Similarly, pulmonary fibrosis is associated with increased lung cancer risk, and chronic pancreatic fibrosis can pave the way for pancreatic adenocarcinoma. The key point is that fibrosis transforms the local environment in ways that make malignant transformation more likely.
3. How does the tumor microenvironment influence cancer progression?
Think of the tumor microenvironment (TME) as the soil in which cancer grows. It includes immune cells, blood vessels, fibroblasts, and extracellular matrix—all of which interact with cancer cells in complex ways. When this environment becomes fibrotic, it can physically shelter tumors from immune attack and therapy, supply them with growth signals, and promote invasive behavior. It’s not just a passive backdrop—it’s an active participant in the cancer’s evolution.
4. What are Cancer-Associated Fibroblasts (CAFs) and their role in cancer?
CAFs are specialized fibroblasts that have been reprogrammed by cancer cells—or sometimes by chronic injury and inflammation—to support tumor growth. They produce pro-growth cytokines, remodel the extracellular matrix, and create biochemical barriers that make tumors more resistant to treatment. In many tumors, CAFs are so abundant they outnumber the cancer cells themselves. They’re no longer considered supporting characters; they’re co-authors of the malignant script.
5. Which organs are most affected by fibrosis-related cancers?
The liver, lungs, and pancreas lead the list—each with well-established pathways from fibrosis to malignancy. The breast is another important site, particularly in women with high mammographic density (which reflects fibrotic tissue composition). The kidneys, prostate, and esophagus also show patterns where fibrotic processes appear to raise cancer risk. Each organ, however, has its own unique mechanisms and progression patterns.
6. How is fibrosis detected in cancer patients?
Detection methods vary. Imaging—like MRI, CT, and ultrasound elastography—can reveal changes in tissue stiffness and structure. Blood biomarkers (such as procollagen peptides, TGF-β, and galectin-3) are under active investigation. Biopsy and histopathology remain the gold standard, allowing for direct visualization of fibrotic tissue. In some cases, emerging tools like AI-enhanced radiomics may soon provide even earlier, more sensitive detection.
7. Are there treatments targeting fibrosis in cancer therapy?
Yes, and this is a rapidly growing area. Anti-fibrotic drugs such as pirfenidone and nintedanib are being repurposed for cancer contexts. TGF-β inhibitors are in clinical trials, and drugs targeting CAFs or breaking down the fibrotic extracellular matrix (like hyaluronidase-based therapies) are under development. Some strategies aim not to destroy the stroma but to “reprogram” it, turning a hostile microenvironment into one that supports therapy.
8. What is the role of TGF-β in fibrosis and cancer?
TGF-β is a master regulator with a dual personality. In normal tissues, it helps control cell proliferation and immune function. But in chronic disease and cancer, it drives fibrosis, suppresses anti-tumor immunity, and encourages cancer cell invasion. Because of this complexity, therapies that target TGF-β must be timed and delivered carefully to avoid unintended consequences.
9. How does fibrosis affect the efficacy of immunotherapy?
It can significantly reduce it. Dense fibrotic tissue acts as a physical barrier to immune cell infiltration. Beyond that, fibrosis alters the cytokine environment, often skewing it toward immune suppression. This means that even potent therapies like checkpoint inhibitors may have limited effect unless the stroma is modified. That’s why combination approaches—anti-fibrotic agents plus immunotherapy—are gaining attention.
10. Can lifestyle changes reduce the risk of fibrosis and associated cancers?
In some cases, absolutely. Reducing alcohol intake, maintaining a healthy weight, controlling diabetes, and avoiding tobacco can all decrease the likelihood of developing fibrosis, particularly in the liver and lungs. Early treatment of underlying diseases like viral hepatitis or autoimmune conditions can also prevent fibrotic progression. While not every fibrotic pathway is lifestyle-driven, many are—and proactive health management plays a powerful preventive role.
Closing Thoughts
As we reach the end of this deep dive into fibrose cancer, it’s worth pausing to reflect on what all this complexity means—not just in theory, but in practice. Fibrosis and cancer are two of the most formidable challenges in medicine, each difficult to understand, each capable of devastating human lives. When they meet, their interaction can feel like a perfect storm: scar tissue fostering malignancy, tumors co-opting the body’s own repair systems to shield and empower themselves.
But here’s the thing. Complexity isn’t the same as hopelessness. In fact, it’s a call to arms for smarter science, better diagnostics, and more nuanced care.
You might be wondering: With all this intricate biology and interwoven pathology, how do we translate knowledge into better outcomes for patients? The answer lies in embracing the very complexity we’ve explored.
First, we must recognize fibrosis as more than just a side effect or a complication. It’s a dynamic process that deserves attention in its own right, as a predictor, a therapeutic target, and sometimes, even a biomarker of cancer risk. That means integrating fibrosis assessment into routine cancer care, refining our diagnostic tools, and personalizing treatments to address not only the tumor but also its fibrotic microenvironment.
Second, the future of fibrose cancer treatment is inherently multidisciplinary. Oncologists, hepatologists, pulmonologists, radiologists, pathologists, and researchers must collaborate seamlessly. Patients benefit when their care teams communicate clearly, share insights, and coordinate therapies that consider the full biological and clinical context.
Third, research continues to blaze exciting trails. From targeting cancer-associated fibroblasts to harnessing immunotherapy in fibrotic tumors, from genetic and epigenetic explorations to biomaterials and microbiome studies—the frontiers are vast and promising. These efforts reflect a profound shift: from attacking cancer cells in isolation to reshaping the entire tumor ecosystem.
Yet, amidst all this, we must never lose sight of the patient—the individual living with this dual burden. Their experiences, challenges, hopes, and fears remind us that medicine is as much art as science. Managing fibrosis and cancer demands empathy, clear communication, and holistic support alongside cutting-edge treatment.
So, what can you take away from this?
Fibrosis and cancer are locked in a complicated dance. Understanding their steps, their rhythms, and their interactions offers a pathway to interrupting disease progression and improving survival. But more than that, it challenges us to think broadly—to appreciate that illness rarely occurs in isolation and that healing sometimes means addressing the environment as much as the invader.
In the end, this knowledge equips clinicians to provide better care, researchers to innovate smarter therapies, and patients to face their journeys with more clarity and hope.
Thank you for joining me on this comprehensive exploration. If you have questions, curiosities, or insights, don’t hesitate to engage further—because the story of fibrose cancer is still unfolding, and every voice matters.