Do Parasites Cause Breast Cancer? Exploring the Connection
- Understanding Parasites and Their Role in Human Disease
- Can Infections Trigger Breast Cancer? The Broader Context
- Mechanisms by Which Parasites May Contribute to Breast Cancer
- Which Parasites Have Been Implicated in Breast Cancer?
- Epidemiological Evidence: What Population Studies Show
- Comparison of Confirmed vs. Suspected Parasite-Linked Cancers
- Challenges in Establishing Causality
- Can Parasites Affect the Tumor Microenvironment in the Breast?
- Animal Models Exploring Parasite–Cancer Interactions
- Immunological Overlap Between Parasitic Infections and Cancer
- Public Health Perspectives: Risk Communication and Equity
- Can Treating Parasites Lower Breast Cancer Risk?
- Current Limitations in Research and Data Availability
- Potential for Future Therapies and Immunomodulation
- Recommendations for Clinicians and Researchers
- Do Parasites Cause Breast Cancer?
- FAQ: Do Parasites Cause Breast Cancer?
Understanding Parasites and Their Role in Human Disease
Parasites are organisms that live in or on a host and derive nutrients at the host’s expense. They include protozoa, helminths (worms), and ectoparasites like lice and mites. Parasitic infections are common worldwide, particularly in regions with limited sanitation or healthcare infrastructure.
Traditionally, parasites have been linked to gastrointestinal illness, anemia, and tropical diseases—not cancer. However, growing evidence shows that chronic parasitic infections can affect the immune system, induce inflammation, and even alter gene expression. These effects may create a microenvironment conducive to tumor development.
Some parasites, like Schistosoma haematobium and Opisthorchis viverrini, are already recognized as carcinogenic in other organs (bladder and liver, respectively). This raises a logical question: could parasites also contribute to the risk of breast cancer?
Can Infections Trigger Breast Cancer? The Broader Context
To understand the potential role of parasites in breast cancer, we first need to examine how infections in general influence cancer risk. Several pathogens are already classified as carcinogenic. Human papillomavirus (HPV) causes cervical cancer, Helicobacter pylori increases the risk of stomach cancer, and hepatitis B and C can lead to liver cancer.
These pathogens don’t just infect; they alter DNA repair, modulate apoptosis, and drive chronic inflammation—three well-established hallmarks of cancer initiation. Similar pathways have been proposed for certain parasites.
The key mechanisms include:
- Persistent immune activation
- Oxidative stress and DNA damage
- Dysregulation of cytokines and growth factors
- Epigenetic changes in host cells
While breast tissue is not a typical site of parasitic colonization, indirect mechanisms—such as systemic immune modulation—may be at play. Emerging data suggests that chronic parasitism could shape systemic immunity in ways that impair tumor surveillance, allowing breast cancer cells to evade immune control.
Mechanisms by Which Parasites May Contribute to Breast Cancer
Although definitive proof is lacking, several mechanisms have been proposed to explain how parasites might indirectly or directly contribute to breast cancer. These mechanisms are based on findings from both human epidemiology and animal models.
Chronic inflammation is a central pathway. Parasitic infections often lead to long-term immune activation, which results in the release of reactive oxygen and nitrogen species. These molecules can damage DNA and create mutations that lead to cancer. In breast tissue, inflammation-driven angiogenesis and remodeling of the extracellular matrix may also support tumor growth.
Another mechanism is immune suppression. Some parasites secrete proteins that downregulate host immunity, allowing them to persist. This immune dampening could also make it easier for nascent tumor cells to grow unchecked. Additionally, parasites may trigger hormonal imbalances that influence breast tissue, especially in helminth-infected populations where estradiol levels may be altered.
These hypotheses parallel how systemic influences—like chronic digestive inflammation in conditions such as colorectal cancer vs IBS—can impact cancer risk even when the primary symptoms occur elsewhere.
Which Parasites Have Been Implicated in Breast Cancer?
Although the research is still limited, a few parasitic species have been studied in relation to breast cancer:
- Toxoplasma gondii: This intracellular protozoan infects a significant portion of the global population. Some studies suggest that chronic Toxoplasma infection may alter host immunity and increase breast cancer risk, although findings are inconsistent.
- Schistosoma species: While more commonly associated with bladder and liver cancer, Schistosoma-induced immune modulation has been speculated to affect mammary tissue.
- Echinococcus granulosus: Hydatid disease caused by this tapeworm has been found in proximity to breast tumors in rare case reports, but causality remains unproven.
Animal models also offer clues. Mice infected with certain helminths show changes in breast tissue architecture and immune response. These effects, however, are often context-dependent and difficult to extrapolate directly to human pathology.
More robust population-level studies and mechanistic trials are needed to establish whether these organisms play a causal, contributing, or merely coincidental role in breast cancer development.
Epidemiological Evidence: What Population Studies Show
Epidemiological data exploring the connection between parasitic infections and breast cancer is sparse and inconclusive. Some regional studies in parts of Africa, South America, and the Middle East have reported higher breast cancer incidence in areas where parasitic infections are endemic. However, correlation does not equal causation, and these findings are confounded by many variables, including access to healthcare, genetic predisposition, and environmental exposures.
For example, a retrospective study from Egypt observed that women with prior schistosomiasis were more likely to develop breast abnormalities, including cancer, but the sample size and diagnostic standards were limited. A study in Brazil linked Toxoplasma exposure to altered immune profiles in breast cancer patients but stopped short of claiming causality.
Large-scale prospective studies are lacking, and most available data come from small case-control analyses. As such, the field remains in the hypothesis-generating stage. Still, the regional clustering of certain cancers alongside parasitic disease prevalence suggests that further investigation is warranted.
Comparison of Confirmed vs. Suspected Parasite-Linked Cancers
| Parasite | Confirmed Cancer Link | Proposed Link to Breast Cancer | Mechanism Involved |
| Schistosoma haematobium | Bladder cancer | Weak hypothesis, not confirmed | Chronic inflammation, immune modulation |
| Opisthorchis viverrini | Cholangiocarcinoma (bile duct) | None currently reported | DNA damage via nitrosamines |
| Clonorchis sinensis | Bile duct cancer | None | Oxidative stress and chronic epithelial injury |
| Toxoplasma gondii | No confirmed cancer type | Hypothetical link to breast cancer | Systemic immune suppression, hormonal disruption |
| Echinococcus granulosus | No confirmed cancer type | Rare case associations with breast tumors | Space-occupying effects, local inflammation |
Challenges in Establishing Causality
Demonstrating a causal relationship between parasites and breast cancer faces several scientific and methodological challenges. First, the breast is not a typical site of parasitic colonization, making direct mechanisms less plausible compared to organs like the liver or bladder. Second, most parasitic diseases affect immune and metabolic pathways indirectly, which makes attribution more complex.
Temporal association is another issue. Parasitic infections often occur years before cancer diagnosis, making it hard to link exposure with outcome. Many infections are also asymptomatic or go undetected, further obscuring reliable exposure assessment in retrospective studies.
In terms of molecular biology, few studies have directly demonstrated parasite-derived proteins or DNA within breast tumors. This absence limits direct mechanistic evidence, though indirect immune changes remain an area of interest.
Finally, funding for parasite-oncology research is limited. Since parasitic diseases are more prevalent in lower-income regions, they receive less attention in global cancer research priorities, despite their potential impact.
Can Parasites Affect the Tumor Microenvironment in the Breast?
One of the more intriguing hypotheses is that parasites may not need to infect breast tissue directly to influence tumor development. Instead, their systemic effects—especially on immune surveillance—could alter the tumor microenvironment (TME), making it more permissive to cancer growth.
Parasite-induced immune suppression could impair the detection and elimination of early neoplastic cells by T-cells or natural killer cells. Alternatively, chronic activation of macrophages by parasitic antigens could promote a pro-tumor inflammatory environment. This has been observed in experimental models, where parasite-exposed animals develop tumors with more immunosuppressive stromal signatures.
In theory, parasitic infections could also prime certain cytokine pathways—such as IL-10, TGF-β, or TNF-α—that create an environment conducive to tumor growth. These cytokines are known to affect angiogenesis, immune evasion, and fibroblast activation.
This mirrors other discussions of intracellular and extracellular signaling shifts, such as those explored in What is PTP1B in breast cancer?, where intracellular enzymes indirectly reshape how cells interact with their surroundings.
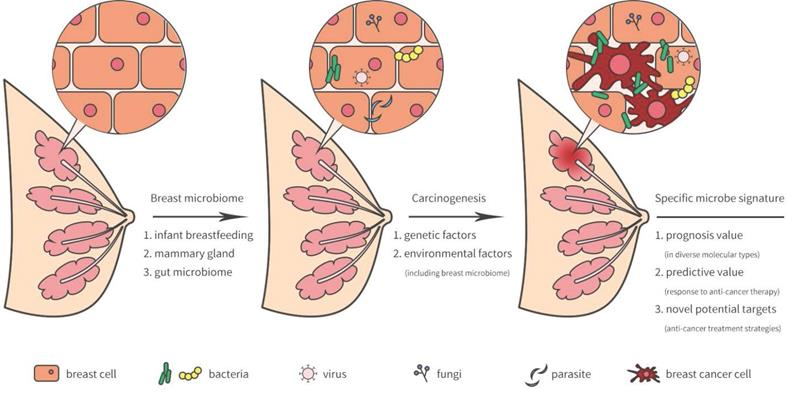
Animal Models Exploring Parasite–Cancer Interactions
Animal studies offer some of the most compelling insights into how parasites might influence breast cancer biology, even if direct translation to human pathology is still limited. In murine models, chronic helminth infection has been shown to alter immune cell distribution and responsiveness. When breast cancer cells are implanted in these animals, tumor growth and metastatic patterns often shift.
In one study, mice with active schistosomiasis developed more vascularized and immunosuppressive breast tumors compared to uninfected controls. Another model involving Toxoplasma gondii exposure suggested that the parasite may prime the immune system in ways that either hinder or facilitate tumor growth, depending on timing and immune status.
While these models don’t prove causation in humans, they validate many of the theoretical mechanisms—especially those related to inflammation, cytokine regulation, and immune surveillance. Future research using genetically modified strains and refined infection protocols may bring even greater clarity to these complex interactions.
Immunological Overlap Between Parasitic Infections and Cancer
Parasitic infections and cancer often trigger overlapping immune responses, such as chronic inflammation, regulatory T-cell activation, and changes in antigen presentation. These parallels may explain why parasitic infections can mimic, mask, or modify cancer progression.
For example, both cancer and helminths can induce expression of IL-10 and TGF-β, leading to suppression of cytotoxic T-cell activity. Similarly, the recruitment of tumor-associated macrophages (TAMs) during cancer mirrors the immune modulation seen during helminth infection. In both cases, the host environment becomes skewed toward tolerance rather than eradication.
Parasites and tumors may also share antigens—or at least cross-reactive epitopes—leading to immune confusion. This is an emerging field of study that may yield new biomarkers for exposure or even targets for immunotherapy. As of now, however, these mechanisms remain speculative and require further molecular validation.
Public Health Perspectives: Risk Communication and Equity
One of the challenges in discussing parasites and breast cancer is avoiding misinformation and stigma. In many parts of the world, parasitic infections are linked with poverty, poor sanitation, or lack of access to healthcare. Suggesting a cancer link without sufficient data can amplify anxiety or misdirect health policy.
At the same time, ignoring potential connections because they predominantly affect marginalized populations perpetuates health inequity. Women in parasite-endemic regions may face dual burdens: limited cancer screening and unrecognized infection-driven risk factors.
Public health messaging should focus on education, preventive care (including deworming and clean water initiatives), and improved access to diagnostic services. Surveillance programs that integrate infectious disease screening with cancer awareness campaigns may help identify trends and generate actionable data.
This approach echoes similar complexities observed in stage 4 neuroendocrine cancer spread to liver life expectancy, where prognosis varies widely based on healthcare access, biology, and early intervention.
Can Treating Parasites Lower Breast Cancer Risk?
This is one of the most important but least studied questions. While it is logical to assume that eliminating parasites might reduce systemic inflammation or restore immune function, no clinical trials have demonstrated that antiparasitic treatment lowers breast cancer incidence.
That said, treating parasitic infections is beneficial in its own right and may have downstream effects on immune health. For example, mass deworming programs improve hemoglobin levels, reduce cytokine load, and enhance vaccine responsiveness. These effects could plausibly reduce cancer risk long-term, even if indirectly.
Future studies may explore whether treated vs. untreated parasitic infections influence rates of breast or other non-endemic cancers. Until then, clinicians should continue to manage parasitic infections per current guidelines and remain alert to the broader health implications of immune modulation in chronically infected individuals.
Current Limitations in Research and Data Availability
Despite growing curiosity about the connection between parasites and cancer, particularly breast cancer, major gaps remain in the scientific literature. Most available data come from case reports, regional studies, and experimental animal models—not from large-scale, peer-reviewed clinical trials. This makes it difficult to draw definitive conclusions or form clinical guidelines.
Funding and research prioritization are also barriers. Since parasitic infections disproportionately affect lower-income countries, there is less commercial or institutional investment in this line of inquiry. Furthermore, cancer registries rarely include infectious disease histories, making retrospective analysis challenging.
Another issue is diagnostic sensitivity. Chronic parasitic infections are often underdiagnosed or misclassified, especially in asymptomatic cases. Without consistent serologic or molecular screening, it’s difficult to assess parasite exposure in breast cancer cohorts accurately.
For the field to advance, better-designed epidemiological studies, standardized parasite screening in oncology trials, and interdisciplinary collaboration between oncologists, immunologists, and infectious disease specialists are essential.
Potential for Future Therapies and Immunomodulation
Even if parasites are not direct carcinogens, they may influence future cancer therapies. Some researchers are investigating whether controlled exposure to parasite antigens or immune products could be used to “re-educate” the immune system in cancer patients.
For example, helminth-derived molecules have been shown to modulate dendritic cell activation, suppress autoimmunity, and balance T-cell responses. These immunomodulatory properties are being explored as adjunct therapies in inflammatory diseases—and may someday be tested in cancer immunotherapy as well.
Although this sounds counterintuitive, it is part of a broader trend of harnessing immune complexity for therapeutic gain. If parasite-derived compounds can reduce tumor-promoting inflammation or restore immune surveillance, they could become valuable tools, even if parasites themselves are not oncogenic.
Such strategies are still theoretical, but they open a new chapter in understanding host–parasite–tumor interactions and leveraging that triad for patient benefit.
Recommendations for Clinicians and Researchers
At present, no changes to breast cancer screening or prevention protocols are recommended based on parasitic infection status. However, clinicians working in endemic regions should remain aware of this research and consider a patient’s infectious disease background when evaluating atypical tumor behavior or immune profiles.
Researchers are encouraged to incorporate parasitic screening into cohort studies, particularly in global cancer research. Partnerships with parasitologists, epidemiologists, and molecular biologists will help bridge the existing gaps and move the field from speculation to science.
Medical educators should also consider integrating emerging evidence on infection-driven carcinogenesis—including parasitic factors—into oncology curricula. Doing so may help reduce geographic and disciplinary bias in the understanding of cancer causes and risk factors.
Do Parasites Cause Breast Cancer?
The short answer is: not directly, based on current evidence. However, the systemic effects of parasitic infections—chronic inflammation, immune suppression, and hormonal modulation—may contribute to a pro-tumor environment under the right conditions.
The relationship between parasites and breast cancer remains an open question worthy of further investigation. While definitive proof is lacking, enough biological plausibility exists to justify deeper study, particularly in endemic areas where both parasitic diseases and breast cancer are prevalent.
Understanding how external infections shape internal risk will help advance not only cancer biology but also global health equity. Until more is known, the prudent approach is to continue standard breast cancer prevention and treatment while staying curious about the hidden connections that may one day rewrite what we think we know.
FAQ: Do Parasites Cause Breast Cancer?
Can parasites directly infect breast tissue?
In most cases, no. The breast is not a common site for parasitic colonization, though rare cases of localized cysts or abscesses from parasitic infections have been reported.
Is there scientific proof that parasites cause breast cancer?
No conclusive proof exists. Some studies suggest a possible link through immune system changes, but more research is needed.
Which parasites have been studied in relation to breast cancer?
Toxoplasma gondii, Schistosoma species, and Echinococcus granulosus have been examined, but none have been definitively linked to breast cancer development.
How could parasites theoretically increase cancer risk?
They may cause chronic inflammation, suppress the immune system, or alter hormone levels, all of which could support tumor growth over time.
Do any parasites cause cancer in other organs?
Yes. Schistosoma haematobium is linked to bladder cancer, and Opisthorchis viverrini is linked to liver bile duct cancer.
Could eliminating parasites lower breast cancer risk?
There is no direct evidence, but reducing chronic infection may improve overall immune health, which could indirectly reduce cancer risk.
What role does inflammation play in this connection?
Long-term inflammation from persistent parasitic infections can create a microenvironment that fosters cellular damage and mutations.
Are there breast cancer cases caused by Toxoplasma gondii?
There are case reports suggesting a correlation, but not causation. More controlled studies are needed.
Can parasite infections mimic cancer symptoms?
Yes. Some parasitic diseases can cause masses, pain, or systemic symptoms that may be mistaken for tumors.
Should breast cancer patients be screened for parasites?
Not routinely, unless they are from endemic regions or have symptoms suggestive of parasitic infection.
Are any antiparasitic drugs used in cancer treatment?
Some are under investigation for their immune-modulating effects, but they are not standard treatments in oncology.
What’s the link between immune suppression and cancer?
When the immune system is suppressed—whether by parasites or other causes—it may fail to detect and destroy emerging cancer cells.
Do parasites affect cancer treatment outcomes?
Potentially. Immunosuppression from chronic infection might alter response to chemotherapy or immunotherapy, but more data is needed.
Is there a vaccine to prevent parasite-linked cancers?
Not currently. Some vaccines exist for parasites (e.g., malaria), but none are designed for cancer prevention.
What can be done today based on current knowledge?
Focus on general health: treat parasitic infections, follow standard cancer screening guidelines, and stay informed as new research emerges.