COVID-19: The Evolving Pandemic Landscape
Introduction
The COVID-19 pandemic—can you believe it’s been several years since it first upended our world? What began as a cluster of mysterious pneumonia cases in Wuhan, China, quickly snowballed into a global health emergency unlike anything we’ve seen in modern history.
And mutations here can affect how well the virus enters cells, evades immune responses, or resists vaccines. We’ve seen similar evolutionary strategies in Avian Influenza H5N1, a virus whose ability to mutate and shift antigenically has complicated vaccine development efforts for decades—offering lessons that still echo in our current strategies against COVID-19.
But what exactly made this virus so disruptive, and why did it manage to bring entire countries, economies, and healthcare systems to their knees? Reflecting on past viral threats—like the Marburg virus, which similarly triggered global concern due to its high fatality rate—helps us frame COVID-19 not as an anomaly, but as part of a broader continuum of emerging infectious diseases we’ve long underestimated.
COVID-19, caused by the novel coronavirus SARS-CoV-2, was officially declared a pandemic by the World Health Organization in March 2020. In the months that followed, we saw unprecedented measures taken across the globe: borders closed, cities locked down, schools and businesses shuttered, and face masks became as essential as car keys. But behind the statistics and news headlines were very real human stories—of loss, resilience, adaptation, and scientific triumph.
So, how did a single virus change the way we live, work, and interact with each other? And what have we learned, not just scientifically but socially and emotionally, from this global experience?
From a public health perspective, the impact of COVID-19 has been staggering. Millions of lives were lost, and many more were altered by severe illness, long-term complications, or the socioeconomic fallout of the pandemic. Healthcare systems were overwhelmed, exposing longstanding gaps in infrastructure and resource allocation. But it wasn’t just hospitals that were affected—every corner of society felt the ripple effect. The global economy shrank, supply chains fractured, and unemployment rates soared in many regions.
Yet amidst the chaos, the pandemic also sparked incredible innovation and collaboration. Scientists across continents worked together at record speed to decode the virus, develop diagnostic tools, and roll out vaccines in under a year—a process that typically takes a decade or more. Isn’t that remarkable?
Now, in 2025 and beyond, we find ourselves in a new phase of the pandemic. COVID-19 hasn’t disappeared, but it has evolved—and so have we. Our understanding of the virus, its transmission, and its long-term effects continues to grow. Public health strategies are adapting, new treatments are being developed, and vaccination efforts remain a central focus.
So, where do we stand now? And how do we prepare for what comes next?
In this chapter, we’ll explore the virus itself, how it affects the body, how we diagnose and treat it, and what the future might hold. Whether you’re a healthcare professional, a student, or someone simply trying to make sense of it all, this deep dive will aim to provide both clarity and context on the evolving landscape of COVID-19.
Virology and Pathogenesis
Let’s start with the virus itself. What is SARS-CoV-2, really? How does it work? Why did it spread so fast, and why is it still evolving?
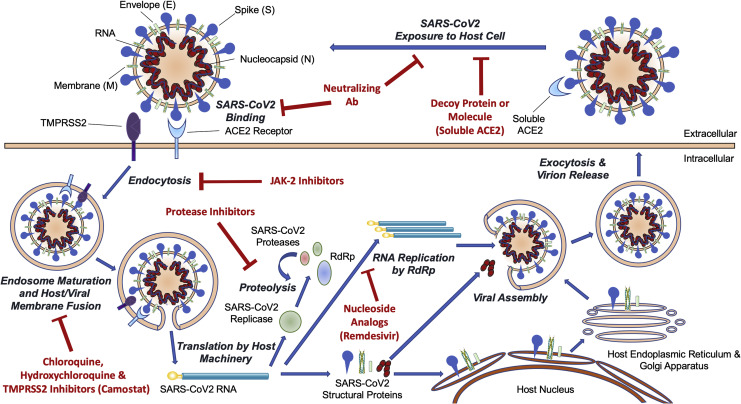
SARS-CoV-2 stands for “Severe Acute Respiratory Syndrome Coronavirus 2.” Sound familiar? That’s because it’s part of the same virus family that caused the SARS outbreak in 2002–2003. But this one has some crucial differences. For starters, it’s far more contagious—and that’s a big part of the problem. While SARS-CoV-1 was mostly contained, SARS-CoV-2 spread across the globe in a matter of months. But why?
Structure of SARS-CoV-2
At its core, SARS-CoV-2 is a single-stranded RNA virus. Imagine a tiny ball wrapped in spikes—those famous “spike proteins” you’ve probably seen in countless diagrams and news reports. These spike proteins are the key to the virus’s success. Literally.They bind to a receptor on human cells called ACE2, which is found in the lungs, heart, intestines, and other tissues. Think of ACE2 like a doorknob, and the spike protein as the key. Once the virus “unlocks” the door, it enters the cell and hijacks the host’s machinery to start making copies of itself. And just like that, infection begins.
So what’s the big deal about this structure? One word: adaptability. The spike protein is also the part that mutates most frequently. And mutations here can affect how well the virus enters cells, evades immune responses, or resists vaccines. We’ve seen similar evolutionary strategies in Avian Influenza H5N1, a virus whose ability to mutate and shift antigenically has complicated vaccine development efforts for decades—offering lessons that still echo in our current strategies against COVID-19.
Mechanisms of Infection and Mutation Patterns
The pathogenesis—or how the disease develops—starts almost immediately after infection. After entering the body, the virus begins replicating, usually in the upper respiratory tract. In many cases, the immune system kicks in and clears it out quickly, resulting in mild symptoms or none at all. But in some people, the immune response becomes exaggerated and dysregulated. Ever heard of the “cytokine storm”? It’s an overreaction by the immune system that can cause inflammation, tissue damage, and even organ failure.
Why does this happen in some people and not others? That’s one of the most puzzling aspects of COVID-19. Age, underlying health conditions, genetics, and even gut microbiota may play a role—but the exact interplay is still being studied.
Now, let’s talk about mutations. Why do they keep happening? Because RNA viruses like SARS-CoV-2 replicate quickly and aren’t great at proofreading. Each time the virus replicates, there’s a chance for a “typo” in its genetic code. Some typos do nothing. Some make the virus weaker. But every now and then, a mutation gives the virus an edge—say, by helping it spread more easily or evade antibodies. And that’s how we end up with new variants like Delta, Omicron, and the newer lineages that keep emerging.
Here’s something to think about: Is the virus getting stronger, or are we just getting better at detecting and responding to it? In reality, it’s a bit of both.
Understanding the virology and pathogenesis of SARS-CoV-2 helps explain why it has such a wide range of effects—from a mild cold to severe respiratory distress and beyond. It also underscores the importance of vaccines, antiviral drugs, and public health interventions that aim to interrupt the virus at various points in this process.
So next time you hear about a “variant of concern” or a breakthrough in treatment, you’ll have a better sense of what’s happening at the microscopic level. Because the virus may be invisible, but its impact is anything but.
Clinical Presentation
When someone says they’ve “caught COVID,” what does that actually mean? Are we talking about a sniffle, a fever, a week in bed—or something far more serious? One of the most complicated things about COVID-19 is that its clinical presentation isn’t one-size-fits-all. It’s a bit of a chameleon, capable of producing everything from a completely silent infection to life-threatening illness.
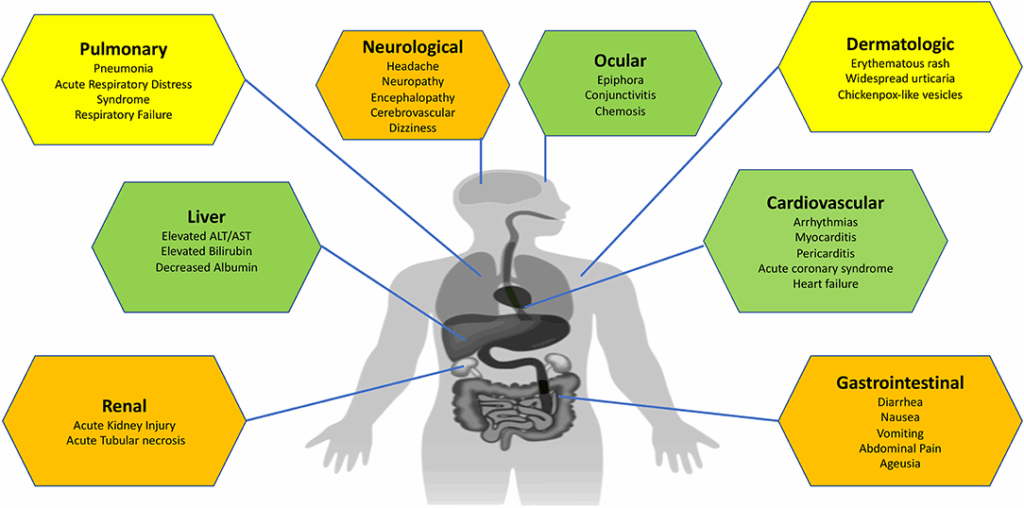
So, what does COVID-19 look like in real life? And why does it hit some people so much harder than others?
Range of Symptoms from Mild to Severe
Let’s start with the basics: symptoms. The most common ones include fever, cough, fatigue, and loss of taste or smell. But that’s really just the tip of the iceberg. People have also reported sore throat, body aches, gastrointestinal symptoms like diarrhea or nausea, and even neurological signs such as confusion or “brain fog.” Some folks have a single mild symptom that lasts a day or two. Others end up in the ICU.
Why such a wide range? It depends on several factors: the viral load (how much virus you’re exposed to), your age, your immune system, any underlying conditions, and—yes—pure chance. It’s not always predictable. Two people in the same household might have vastly different experiences with the same variant.
Another wrinkle: timing. COVID-19 symptoms can appear anywhere from 2 to 14 days after exposure. Some people feel fine for days and then suddenly crash. Others experience a steady decline, or bounce back only to relapse later. It’s not just a disease—it’s a moving target.
And don’t forget asymptomatic cases. Did you know that some people never develop any symptoms at all but can still spread the virus to others? That’s part of what made this pandemic so hard to contain.
Risk Factors for Severe Disease
Now, who’s most at risk for developing severe COVID-19? You’ve probably heard the usual list: older adults, people with chronic conditions like diabetes, heart disease, or obesity, and those who are immunocompromised. But it’s worth diving deeper into why.
Age is a major factor. As we age, our immune systems naturally weaken—a process called immunosenescence. This means older adults are less equipped to mount a strong response against the virus. But age alone doesn’t tell the whole story. Plenty of younger people with no obvious health issues have also ended up severely ill.
So what else contributes to risk? Think about underlying inflammation and vascular health. COVID-19 isn’t just a respiratory disease—it affects the blood vessels, the heart, the kidneys, and the brain. It can cause blood clots, strokes, heart attacks, and even multisystem inflammatory syndromes.
And here’s a critical point: social determinants of health also play a huge role. People in underserved communities—especially those with less access to healthcare, safe housing, and nutritious food—have been disproportionately affected. So when we ask, “Who is most at risk?” the answer isn’t just biological. It’s social. It’s economic. It’s systemic.
Have you ever wondered why some people recover quickly while others face a long, complicated road to recovery? We’re still trying to understand the full picture, but research is ongoing into genetic susceptibility, immune system variability, and even preexisting levels of inflammation.
All of this highlights a key truth: COVID-19 is not just a respiratory infection. It’s a multi-system disease with unpredictable outcomes. That’s why even seemingly “mild” cases deserve careful monitoring—and why public health efforts focus on prevention, not just treatment.
Diagnosis
How do you know if you actually have COVID-19? It sounds like a simple question, right? You just take a test. But as anyone who’s lived through the pandemic can tell you, it’s a bit more complicated than that.
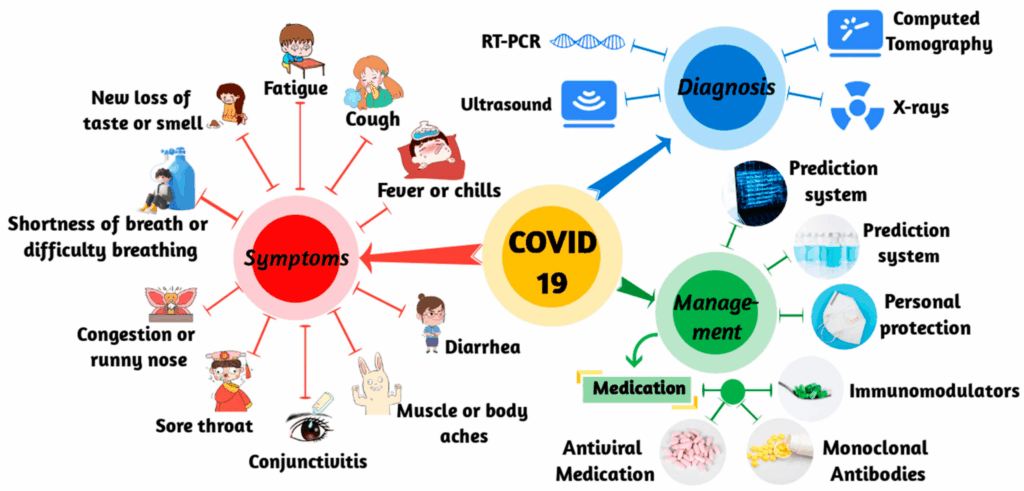
From the early days of the pandemic to the latest waves and variants, testing has played a central role in both individual decisions and global public health policy. But how reliable are these tests? What do they really tell you—and what can they miss?
Let’s dig into how we diagnose COVID-19, the types of tests available, and why, even now, testing remains both essential and occasionally frustrating.
Testing Methods: PCR, Antigen, and Antibody Tests
The gold standard for diagnosing an active COVID-19 infection has long been the PCR test—short for polymerase chain reaction. What makes PCR so accurate? It detects the virus’s genetic material—its RNA—with incredible sensitivity. A PCR test can catch an infection even when the viral load is low, which is crucial for early detection. The downside? It takes time. Samples need to be processed in a lab, which often means waiting 24–72 hours for results (though rapid PCR tests are now more common in clinics and airports).
Then there’s the rapid antigen test—the over-the-counter kind you can use at home. You’ve probably seen or taken one of these by now. It’s quick, easy, and gives results in about 15 minutes. But here’s the catch: it looks for proteins on the surface of the virus, not its genetic material. That means it’s less sensitive, especially early in the infection or if the viral load is low. So a negative result doesn’t always mean you’re in the clear.
Ever wonder, “Can I trust this negative test?” That depends on a lot of things—when you took it, how well you swabbed, how soon after exposure you tested, and which test you used. This is why health professionals often recommend multiple tests over several days if symptoms persist or exposure is confirmed.
And what about antibody tests? These aren’t used to detect current infections. Instead, they look for past exposure by detecting antibodies—proteins your immune system creates in response to the virus. They can help answer questions like, “Did I already have COVID and not realize it?” or “Did the vaccine trigger an immune response?” However, antibodies can fade over time, and these tests don’t give the full picture of your immunity.
So, are tests perfect? No. But they’re still among our best tools for managing outbreaks and making informed decisions. Which brings us to…
Challenges in Detection and False Negatives
If you’ve ever had symptoms, taken a test, and gotten a negative result—only to test positive a day later—you’re not alone. COVID-19 testing has always come with a certain amount of uncertainty. Why? Because timing is everything.
There’s a window—usually a few days after exposure—when the virus is incubating but hasn’t reached detectable levels yet. Test too early, and you may get a false negative. Test too late, especially with an antigen test, and the virus may have already moved deeper into the lungs or begun to decline in the nose and throat where you swab. That’s why repeat testing and context (symptoms, exposure history) are so important.
Also worth noting: sample quality matters. Poor swabbing technique can lead to an invalid result or false reassurance. Think of it this way—if the test is trying to detect something microscopic, getting a good sample is like trying to collect morning dew with a spoon. It takes precision.
And then there’s variant detection. Some variants can slightly alter the virus’s structure in ways that interfere with certain tests, especially those that aren’t updated or calibrated for the newer strains. Thankfully, most high-quality PCR tests target multiple regions of the virus’s genome, reducing the risk of a variant “escaping” detection entirely.
So, where does that leave us?
The takeaway is this: testing is an evolving science. It’s not perfect, but it’s incredibly useful when interpreted in context. It’s a bit like reading a weather forecast—it tells you a lot, but not everything. Knowing the type of test, when to take it, and how to interpret the result is key to making informed decisions about isolation, treatment, or returning to work or school.
And here’s a question to carry forward: now that we’ve gotten so good at rapid diagnostics, how can we use that knowledge to improve testing for other diseases in the future?
Treatment
Once someone tests positive for COVID-19, the next question is usually: Now what? Is it rest and fluids at home? A trip to the emergency room? Antiviral pills? Oxygen support? The answer, as with so much else in this pandemic, depends on a host of variables—timing, severity, underlying conditions, and access to care.
So, how do we treat COVID-19? And how has our approach evolved from the chaotic early days of the pandemic to the more nuanced strategies we use today?
Antiviral Medications and Supportive Care
Let’s rewind to 2020 for a moment. Back then, treatments were largely supportive. In mild cases, that meant over-the-counter medications for fever, staying hydrated, and monitoring symptoms. In severe cases, patients needed oxygen or ventilation. There were no specific antivirals proven to work against SARS-CoV-2 yet—doctors were essentially flying blind, trying everything from hydroxychloroquine to convalescent plasma, with mixed (and sometimes dangerous) results.
Fast forward to now, and the landscape looks very different.
One of the major breakthroughs has been the development of antiviral drugs that specifically target SARS-CoV-2. Take Paxlovid (nirmatrelvir/ritonavir), for example. It works by inhibiting the virus’s ability to replicate inside human cells. If given early—ideally within five days of symptom onset—it can significantly reduce the risk of hospitalization and severe illness, especially in high-risk individuals. Another antiviral, remdesivir, is administered intravenously and was one of the first drugs shown to speed up recovery in hospitalized patients.
Are these treatments cures? Not quite. But they are critical tools that can change the trajectory of the disease, especially when given early and to the right patients.
That said, not everyone needs—or qualifies for—antivirals. Many people, particularly the young and healthy, can recover with supportive care alone. That includes rest, hydration, fever reducers like acetaminophen, and monitoring for warning signs like shortness of breath, persistent chest pain, or confusion. Telemedicine has become an important resource here, allowing patients to stay home while still being monitored by healthcare providers.
But what happens when the disease becomes severe?
Management of Complications
For hospitalized patients, especially those with low oxygen levels, treatment becomes more aggressive. Oxygen therapy is often the first step. This can range from a nasal cannula (the little tubes that go under your nose) to high-flow oxygen machines or even mechanical ventilation in critical cases.
And here’s where things get even more interesting—and complex. COVID-19 doesn’t just attack the lungs. It can trigger systemic inflammation, leading to blood clots, heart damage, kidney failure, and neurological issues. That’s why treatment may also include anticoagulants (to reduce clotting), steroids like dexamethasone (to calm the immune system), and immunomodulators like tocilizumab (which block specific inflammatory pathways).
So yes, COVID-19 is a respiratory virus—but in severe cases, it behaves more like a whole-body illness. And treating it often requires a team approach: pulmonologists, infectious disease specialists, cardiologists, nephrologists, and more. It’s a reminder of how deeply interconnected our body systems are—and how a virus can exploit those connections in unpredictable ways.
Have we gotten better at saving lives? Absolutely. Mortality rates have dropped significantly since the early days of the pandemic, thanks to a better understanding of the disease and more targeted therapies. But treatment is still a race against time, and access remains uneven. Not everyone has a hospital nearby with ICU beds and trained staff. Not everyone can afford antivirals. And that’s a major challenge moving forward.
So, the real question is: how do we make these treatments equitable and accessible—not just in wealthy nations, but everywhere?
The next frontier in COVID-19 care isn’t just about better drugs; it’s about smarter systems, faster delivery, and more inclusive healthcare infrastructure.
Vaccination
Let’s talk about what many people consider the single most powerful tool we’ve had against COVID-19: vaccines.
Remember the sheer urgency back in 2020? The whole world was holding its breath, wondering when—not if—we’d get a vaccine. There was skepticism, excitement, fear, and above all, hope. And then, faster than most people thought possible, the first vaccines rolled out. But how exactly do they work? Why do we need boosters? And why, even now, is vaccine hesitancy still such a formidable barrier?
Types of Vaccines and Their Efficacy
Let’s begin with the basics: what kinds of COVID-19 vaccines are out there? You might’ve heard of mRNA vaccines like Pfizer-BioNTech and Moderna. These were the trailblazers. Instead of using a weakened virus like traditional vaccines, mRNA vaccines deliver a set of instructions—genetic blueprints—that teach your cells to produce a harmless piece of the virus (specifically, the spike protein). Your immune system sees this foreign protein, mounts a response, and voilà: immunity is born. No virus required.
And then there are viral vector vaccines like AstraZeneca and Johnson & Johnson, which use a harmless adenovirus as a delivery vehicle for the spike protein gene. They work similarly in principle, just via a different route. Inactivated virus vaccines (like Sinopharm or Covaxin) and protein subunit vaccines (like Novavax) are also part of the mix, offering different risk-benefit profiles and logistical considerations, especially in countries with less robust cold storage infrastructure.
So which one is “best”? That depends. mRNA vaccines showed extremely high efficacy in preventing severe disease and death, especially in the early variants. But newer strains have challenged all of them to some degree. Vaccines don’t make you invincible—but they drastically reduce the odds of severe illness, hospitalization, and long COVID. That’s not just statistically significant—it’s life-saving.
Ever wonder why people still get COVID even after being vaccinated? That’s where the concept of breakthrough infections comes in. No vaccine offers 100% protection, especially against a virus that mutates quickly. But it’s a bit like wearing a seatbelt. You might still get into a car accident, but you’re much more likely to walk away unharmed.
Booster Doses and Public Health Strategies
Let’s talk boosters. Why do we need them? The short answer: immunity fades over time. Even the best vaccines don’t provide lifelong protection against a virus that’s constantly evolving. Booster shots “remind” your immune system to stay alert—and they often include updated formulas to better match circulating variants.
Some people have gotten one booster. Others, two or more. High-risk individuals, like the elderly or immunocompromised, may be on a more frequent booster schedule. It’s not about over-vaccination—it’s about staying ahead of the curve. Think of it like updating your antivirus software: you don’t install it once and forget it forever. The threat evolves, and so must the protection.
But vaccines are only part of the story. Public health strategies—such as mass vaccination campaigns, mobile clinics, public education, and mandates—have played a critical role in uptake. Some efforts worked brilliantly; others ran into fierce resistance. And that brings us to one of the thorniest issues of the pandemic: vaccine hesitancy.
Why do some people remain skeptical or outright opposed to vaccination, even when the data is clear? The answers are complex. Distrust in institutions. Misinformation. Cultural or religious beliefs. Political identity. Personal experience. The list goes on. But the effects are tangible: lower vaccination rates mean higher transmission, more hospitalizations, and more room for new variants to emerge.
So, how do we build trust? How do we reach people who are hesitant, not with judgment, but with empathy, facts, and transparency?
That’s a question public health officials—and communities everywhere—continue to grapple with. Because at the end of the day, a vaccine isn’t just a vial of liquid. It’s a tool that only works when it’s used, and when it’s trusted.
Vaccination isn’t the final chapter of the COVID story—but it’s a turning point. One that transformed fear into action, science into protection, and possibility into prevention.
Long COVID
What if the virus doesn’t leave when the fever fades and the cough clears? What if “recovery” isn’t the end of the story—but the beginning of a new and confusing chapter? That’s the reality for millions of people around the world who are living with what’s now widely known as Long COVID.
You may have heard the term before, sometimes called post-acute sequelae of SARS-CoV-2 infection (or PASC), but what does it actually mean? Is it a single illness, a collection of symptoms, or something else entirely? And perhaps most frustratingly: why does it happen to some people and not others?
Definition and Prevalence
Let’s start by defining it. Long COVID refers to a range of symptoms that persist for weeks or months after the initial infection has cleared—typically 4 weeks or more, though many cases stretch far beyond that. It doesn’t seem to matter whether the initial case was mild or severe. In fact, many long COVID sufferers report only mild illness in the acute phase, only to face an avalanche of problems weeks later.
The symptoms are incredibly diverse—more on that in a moment—but what’s crucial to understand is that long COVID is not rare. Estimates vary depending on the study and the population, but it’s generally accepted that at least 10–30% of people who recover from COVID-19 may develop lingering symptoms. That’s a staggering number, when you consider how many people have been infected globally.
And this raises important questions: What’s the biological basis? Is it an autoimmune response, viral remnants hiding in the body, or damage from the acute phase that takes a long time to heal? The truth is, we’re still figuring it out. And that uncertainty can be maddening—for patients and doctors alike.
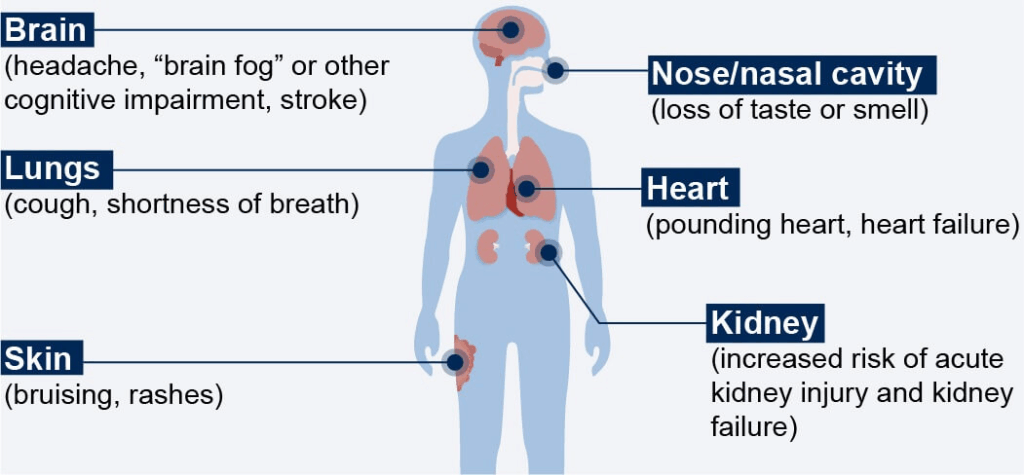
Symptoms and Impact on Quality of Life
So, what does Long COVID feel like? Here’s where things get complicated. The list of reported symptoms is long, and they can vary dramatically from person to person. The most commonly reported include:
- Extreme fatigue that doesn’t improve with rest
- Brain fog—trouble concentrating, forgetfulness, or feeling mentally “fuzzy”
- Shortness of breath or chest tightness
- Heart palpitations or racing pulse
- Muscle and joint pain
- Loss of taste or smell (or unusual distortions of these senses)
- Sleep disturbances
- Anxiety and depression
- Dizziness or imbalance
What’s striking is that many of these symptoms overlap with chronic illnesses like ME/CFS (myalgic encephalomyelitis/chronic fatigue syndrome), POTS (postural orthostatic tachycardia syndrome), and fibromyalgia. This has opened up new conversations in the medical world about how we define and respond to post-viral syndromes—not just for COVID, but potentially for other viruses as well.
Can you imagine having to explain to your boss that even though you “recovered” from COVID three months ago, you’re still too exhausted to get through a full workday? Or trying to convince a doctor that your shortness of breath isn’t anxiety, but something real and ongoing? Many Long COVID patients face not only physical challenges but validation challenges—struggling to be heard and believed.
There’s also a growing recognition that Long COVID disproportionately affects certain groups—women, in particular, appear to be more frequently affected. Why? Hormonal differences? Immune response patterns? Again, the research is early, but the patterns are emerging.
And the impact goes beyond the body. Long COVID has forced many people to reduce work hours, take leave, or leave jobs entirely. Relationships, parenting, financial stability—all of it can suffer. It’s not just a medical condition; it’s a life-altering disruption.
So, what’s being done?
Clinics specifically for Long COVID are opening in many places, offering multidisciplinary care—cardiology, neurology, pulmonary care, physical therapy, and mental health support. But access is inconsistent, and treatments are still largely symptomatic. There’s no one-size-fits-all approach—yet.
Here’s a question we’re still asking: How do we build healthcare systems flexible enough to manage complex, chronic, and often invisible conditions like Long COVID? What do we owe to people who did “everything right” but still ended up in this limbo?
What we do know is this: Long COVID is real. It’s widespread. And it demands long-term attention—not just from doctors, but from policymakers, employers, insurers, and society as a whole.
Recent Developments (2025–2026)
Just when we thought we had COVID-19 figured out—vaccines distributed, treatments refined, public health protocols adjusted—it shifted again. Isn’t that how it always goes? If there’s one truth that has held up throughout this pandemic, it’s that nothing stays still for long.
The last couple of years, 2025 and 2026, have brought a new chapter in the pandemic’s timeline. Less panic, perhaps, but no shortage of complexity. So where are we now? Is COVID-19 fading into the background, or are we settling into a new kind of normal where it remains part of the landscape—like the flu, but with a longer shadow?
Emergence of New Variants
Let’s begin with the ever-moving target: variants. Every time the virus spreads, it gets an opportunity to mutate. Most mutations go nowhere—harmless little errors that don’t change much. But occasionally, one gives the virus a boost: greater transmissibility, stronger immune evasion, or better survival in different environments. And that’s how new variants emerge.
In 2025, the dominant global strains were distant cousins of Omicron—still highly transmissible, but with additional tweaks that allowed them to partially escape previous immunity, whether from vaccines or past infection. You might be wondering: How many times can this virus reinvent itself?
The short answer? As long as it has hosts to infect and opportunities to evolve.
But here’s the interesting twist: while new variants continue to emerge, the severity hasn’t necessarily increased. In fact, most recent variants have favored spread over lethality. That’s an evolutionary trade-off. A virus that kills its host too quickly doesn’t spread as easily. This trend is encouraging—but not a guarantee. We can’t rule out the possibility of a future variant that’s both highly contagious and more virulent.
So scientists are watching closely. Global genomic surveillance programs—basically giant international virus-watching networks—are now standard tools in the public health arsenal. They help us catch new variants quickly, assess risk, and update vaccines if needed.
But it all leads to a bigger question: Are we prepared to pivot quickly enough the next time the virus changes the game?
Updates on Vaccine Development and Distribution
Speaking of pivoting, let’s talk about vaccines—again. They’ve been evolving too.
Gone are the days when mRNA vaccines were revolutionary; now, they’re the new standard. Manufacturers have developed variant-specific boosters targeting the most recent strains, similar to how the flu shot is updated annually. And they’re getting better—more refined, more durable, and in some cases, easier to store and administer.
We’ve also seen exciting movement in the realm of next-generation vaccines:
- Nasal sprays that stimulate mucosal immunity, targeting the virus where it first enters the body—your nose and throat.
- Pan-coronavirus vaccines that aim to protect not just against SARS-CoV-2, but against future coronaviruses as well.
- Self-amplifying RNA platforms, which use smaller doses and could make global distribution more efficient.
These aren’t just scientific flexes—they’re strategic tools in preparing for what’s next. The more flexible and scalable our vaccine technology, the better we can adapt to whatever curveball the virus throws next.
But here’s where things get tricky: distribution and uptake. While wealthier nations have updated their vaccine stockpiles and rolled out seasonal boosters, many low- and middle-income countries are still playing catch-up. Vaccine fatigue and skepticism are also real, even in well-resourced areas. Uptake rates have dropped as people perceive COVID-19 as “over,” even though the virus itself disagrees.
And this raises a difficult question: How do you maintain public engagement and political will when the sense of urgency fades—but the risk hasn’t?
The global response is now moving into the territory of endemic management—treating COVID as a long-term presence rather than a crisis event. This includes:
- Regular boosters for vulnerable populations
- Integration of COVID testing and treatment into routine care
- Surveillance systems that can catch outbreaks early
- And yes, public messaging that walks a fine line between realism and reassurance
There’s no going back to “before.” But that’s not necessarily a bad thing. What’s emerging is a more resilient infrastructure for dealing with infectious disease—not just COVID, but future threats too.
So, where do we go from here?
We’ve learned that managing a pandemic isn’t just about science—it’s about timing, equity, communication, trust, and the strength of the systems we build around that science. The virus may continue to evolve, but so can we. In fact, the ongoing challenge of antiviral resistance—highlighted by cases like H1N1 with drug resistance—reminds us that adaptation isn’t just viral; it’s also pharmacological. Our response must be just as agile, with research, policy, and infrastructure evolving in parallel.
Frequently Asked Questions (FAQ)
1. What made SARS-CoV-2 so disruptive compared to other viruses?
SARS-CoV-2 combined high transmissibility with the ability to spread asymptomatically, making containment incredibly difficult. It also triggered severe disease in a subset of patients and strained healthcare systems worldwide.
2. How does SARS-CoV-2 infect human cells?
It uses its spike proteins to bind to ACE2 receptors on human cells—like a key fitting into a lock—allowing it to enter the cell and hijack its machinery to replicate.
3. Why does COVID-19 present so differently in different people?
Symptoms can range from none at all to life-threatening complications due to factors like viral load, genetics, immune response, age, underlying health conditions, and social determinants.
4. Can you still spread the virus if you don’t have symptoms?
Yes, asymptomatic individuals can still transmit SARS-CoV-2, which is one of the major reasons it spread so rapidly worldwide.
5. How reliable are COVID-19 tests?
PCR tests are very accurate but slower. Rapid antigen tests are faster but less sensitive, especially in early or late stages of infection. Timing and technique matter a lot in getting accurate results.
6. What are the main treatments for COVID-19 today?
Early-stage treatment may involve antiviral drugs like Paxlovid. Severe cases may require oxygen therapy, corticosteroids, anticoagulants, and specialized hospital care for complications.
7. Why do we need COVID-19 booster shots?
Immunity from vaccines wanes over time, and new variants can partially evade older immune responses. Boosters help refresh protection and adapt to circulating strains.
8. What is Long COVID and who gets it?
Long COVID refers to persistent symptoms lasting weeks or months after the initial infection. It can affect anyone—regardless of initial illness severity—and symptoms vary widely, often impacting quality of life.
9. Are new COVID-19 variants still emerging?
Yes, as long as the virus continues to circulate, it mutates. Most new variants are more transmissible, though not always more severe, and scientists monitor them closely for changes that could affect public health.
10. What are the latest developments in COVID-19 vaccine technology?
Recent innovations include nasal vaccines, variant-targeted boosters, and broad-spectrum or “pan-coronavirus” vaccines designed to prepare us for future outbreaks.
11. How do we keep people engaged in COVID safety when the emergency phase feels over?
Public health now focuses on endemic management, trust-building, and integrating COVID care into routine systems, while continuing to educate and prepare the public for potential new surges.
Conclusion
So, after all we’ve lived through, what have we learned?
COVID-19 didn’t just challenge the world medically—it tested our resilience, our cooperation, our capacity for innovation, and, in some ways, our humanity. It exposed vulnerabilities in healthcare systems, inequalities in access, and cracks in the social contract. But it also accelerated scientific breakthroughs, created new forms of remote work and education, and showed what global collaboration can achieve when the stakes are high.
One of the key takeaways? Preparedness isn’t a luxury—it’s a necessity. The ability to detect, respond to, and communicate about emerging threats is no longer optional in a world as interconnected as ours.
Another lesson: science is dynamic. What we “knew” about COVID-19 in 2020 looks very different from what we understand now—and that’s okay. Adapting our policies and expectations based on new data isn’t weakness; it’s wisdom.
And perhaps most importantly: people matter. The stories behind the statistics—the exhausted nurses, the grieving families, the researchers working overnight shifts, the long COVID patients still seeking answers—are what truly define this pandemic.
So where do we go from here?
We move forward, eyes open. We strengthen global health infrastructure. We invest in science. We build public trust. We prepare for the next pathogen—not with fear, but with the lessons of experience.
COVID-19 is still with us, but so is a roadmap for navigating the future—with more clarity, more compassion, and, we hope, more unity than ever before.









