Colon Cancer Metastasis to the Peritoneum: A Professional Guide
- Understanding Peritoneal Metastasis in Colon Cancer
- How Peritoneal Spread Changes the Disease Profile
- Clinical Signs and Patient Symptoms
- Diagnostic Pathways for Confirming Peritoneal Involvement
- Peritoneal Cancer Index (PCI) and Staging
- Treatment Approaches: Systemic and Localized Options
- Role of HIPEC: Mechanism and Indications
- Survival Outcomes by Treatment Modality
- Molecular Genetics and Tumor Biology in Peritoneal Spread
- Managing Recurrent Peritoneal Disease
- Role of Biomarkers in Surveillance and Decision-Making
- Nutritional Support and Symptom Management in Advanced Cases
- Palliative Strategies and Quality-of-Life Focus
- Differences Between Peritoneal Metastasis and Other Metastatic Patterns
- Multidisciplinary Approach and Center Specialization
- Early Recognition and Individualized Planning Matter
- Frequently Asked Questions (FAQ)
Understanding Peritoneal Metastasis in Colon Cancer
Peritoneal metastasis occurs when colon cancer cells spread beyond the bowel wall and seed the peritoneum—the membrane that lines the abdominal cavity and envelops abdominal organs. It is considered a form of stage IV disease and carries a guarded prognosis, especially in cases with extensive dissemination.
The process begins with local invasion of the serosal surface by the primary tumor, often through a combination of vascular invasion, lymphatic spread, or direct penetration. Once free tumor cells are in the peritoneal cavity, they can attach to mesenteric surfaces, the omentum, bowel serosa, or pelvic peritoneum. The peritoneal microenvironment, rich in vascular and immune-modulatory elements, supports the implantation and proliferation of these cells.
This pattern of spread is not unique to colon cancer. For example, in medullary thyroid cancer, microcalcifications in imaging are early signs of distant spread via lymphatics, even without mass formation.
How Peritoneal Spread Changes the Disease Profile
Unlike liver or lung metastasis, which may present as discrete nodules, peritoneal metastasis often appears as diffuse or miliary implants. This leads to a different clinical behavior, one that is harder to image, harder to stage, and more complex to treat surgically.
The condition is often accompanied by progressive ascites, bowel obstruction, and cachexia. Peritoneal metastases can also impair gastrointestinal motility by encasing segments of bowel or causing adhesions. The cancer burden tends to be underappreciated on standard scans, which creates challenges for early intervention.
Because the peritoneum is not a single organ but a complex anatomical space, treatments like systemic chemotherapy must overcome physical barriers to reach cancer cells effectively. Cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) has emerged as the most aggressive yet potentially curative option in selected patients.
Clinical Signs and Patient Symptoms
Peritoneal metastasis rarely causes symptoms in its earliest stages. Most signs develop progressively as tumor deposits expand and interfere with peritoneal and gastrointestinal function. Common presenting symptoms include:
- Persistent or unexplained abdominal bloating, often due to ascites
- Diffuse or localized abdominal discomfort
- A sensation of heaviness or fullness after small meals
- Alternating constipation and diarrhea
- Nausea or vomiting from subacute bowel obstruction
- Unintended and progressive weight loss
Some patients report generalized fatigue or a decreased appetite even before significant anatomical distortion occurs. These nonspecific symptoms often mimic benign gastrointestinal disorders, leading to delayed diagnosis.
Diagnostic Pathways for Confirming Peritoneal Involvement
Diagnosis typically begins with contrast-enhanced CT imaging. Radiologists look for signs such as peritoneal thickening, nodularity, scalloping of organ surfaces, omental caking, and free fluid (ascites). However, sensitivity decreases for small-volume disease.
PET/CT and MRI can provide complementary data, especially when evaluating treatment response or planning surgical intervention. Unfortunately, peritoneal metastases are often underestimated even with these modalities.
Diagnostic laparoscopy remains the gold standard for assessing peritoneal carcinomatosis. It allows direct visualization of implants, assessment of the Peritoneal Cancer Index (PCI), and biopsy confirmation. In select patients, cytological analysis of ascitic fluid may also reveal malignant cells, although sensitivity is lower.
Much like how peritoneal nodules may escape detection in early imaging, thyroid malignancies like medullary thyroid cancer may only become apparent after functional symptoms or biomarker elevations are seen, despite the absence of overt mass lesions.
Peritoneal Cancer Index (PCI) and Staging
Staging peritoneal metastasis from colon cancer requires more than traditional TNM classification. The Peritoneal Cancer Index (PCI) is the most widely accepted tool to quantify the extent and distribution of peritoneal disease. It divides the abdominal cavity into 13 regions and scores each region from 0 to 3, depending on the size of tumor implants.
The total PCI score ranges from 0 to 39. Lower scores (≤10) are generally associated with better outcomes following cytoreductive surgery and HIPEC, while higher scores often predict incomplete resection and limited benefit from aggressive intervention.
In combination with radiologic findings, PCI helps surgeons determine operability and plan the extent of dissection. It is especially relevant during diagnostic laparoscopy, where the surgeon can map the disease burden before proceeding with definitive resection.
When interpreted alongside other staging data (e.g., nodal status or liver metastases), PCI offers a more nuanced understanding of prognosis and guides therapeutic sequencing, such as whether systemic therapy should precede or follow local treatment.

Treatment Approaches: Systemic and Localized Options
The management of peritoneal metastasis in colon cancer is highly individualized. The two main strategies are systemic chemotherapy and cytoreductive surgery with HIPEC. Each plays a distinct role, depending on tumor biology, disease spread, and patient performance status.
Systemic chemotherapy remains the first-line treatment for most patients with unresectable or widespread peritoneal disease. Regimens typically include fluoropyrimidines (5-FU or capecitabine) combined with oxaliplatin or irinotecan, with or without targeted agents like bevacizumab or anti-EGFR therapies.
For patients with limited peritoneal spread and good performance status, cytoreductive surgery (CRS) and HIPEC offer a more aggressive, potentially curative option. This involves complete removal of visible tumor followed by perfusion of heated chemotherapy agents directly into the peritoneal cavity. The heat increases drug penetration and cytotoxicity.
However, this approach is not suitable for all. The presence of extensive small bowel involvement, high PCI scores, or comorbidities may preclude safe or effective surgery. Therefore, careful patient selection and preoperative staging are essential.
Interestingly, this combination of real-time imaging, patient-specific targeting, and thermal enhancement has parallels with TULSA-PRO procedures for prostate cancer, where MRI guidance and localized heating are used to treat cancer while preserving healthy tissue.
Role of HIPEC: Mechanism and Indications
HIPEC (Hyperthermic Intraperitoneal Chemotherapy) is a specialized intraoperative treatment used in conjunction with CRS. After all visible peritoneal metastases are surgically removed, the abdominal cavity is bathed with heated chemotherapy—typically mitomycin C or oxaliplatin—circulated for 30 to 90 minutes at 41–43°C.
This technique targets microscopic residual disease and provides higher local drug concentration compared to systemic chemotherapy. The hyperthermia increases membrane permeability and disrupts DNA repair mechanisms in tumor cells.
Indications for HIPEC include:
- Isolated peritoneal carcinomatosis from colorectal origin without extra-abdominal metastases
- PCI score low enough to allow complete cytoreduction
- Good functional status (ECOG 0–1)
- Absence of significant small bowel or mesenteric involvement
Long-term survival data from studies like PRODIGE-7 and others show mixed results, emphasizing the importance of appropriate patient selection and surgical expertise.
Survival Outcomes by Treatment Modality
| Treatment Modality | Median Overall Survival | 5-Year Survival Rate | Key Factors Influencing Outcome |
| Systemic Chemotherapy Only | 12–18 months | <10% | Tumor burden, KRAS/BRAF mutation status, response to chemo |
| CRS + HIPEC (Curative Intent) | 30–60 months | 25–40% | PCI ≤10, R0 resection, no extra-abdominal spread |
| Palliative Surgery | Variable (often <12 months) | <5% | Symptom control focus, not intended for disease eradication |
| Best Supportive Care | 4–8 months | <2% | Reserved for non-operable cases or declining systemic therapy |
The disparity between survival outcomes underscores the critical importance of early diagnosis, comprehensive staging, and multidisciplinary evaluation.
Molecular Genetics and Tumor Biology in Peritoneal Spread
The molecular profile of colon cancer plays a significant role in determining its metastatic behavior, including its tendency to spread to the peritoneum. Key mutations associated with peritoneal dissemination include BRAF V600E, KRAS, and PIK3CA. These genetic alterations are often linked to more aggressive, mucinous, and poorly differentiated histologies, which have a predilection for transcoelomic (peritoneal) spread.
Mismatch repair deficiency (dMMR) or microsatellite instability-high (MSI-H) tumors are less likely to metastasize to the peritoneum but may still do so in specific molecular contexts. Conversely, tumors with chromosomal instability often favor this pattern of spread, particularly in right-sided colon cancers.
Understanding these genetic underpinnings helps clinicians select targeted therapies, such as BRAF inhibitors, and assess suitability for immunotherapy. Furthermore, tumor biology can predict response to HIPEC — for example, mucinous carcinomas with signet-ring features may respond poorly and progress rapidly.
The concept of biologically aggressive yet physically subtle disease is not unique to colon cancer. In breast cancer without a lump, for instance, biology often dictates the course of disease more than tumor size.
Managing Recurrent Peritoneal Disease
Even after complete cytoreduction and HIPEC, recurrence is a significant challenge. Peritoneal relapse can occur due to microscopic residual disease, particularly in areas that are technically difficult to access, such as the mesentery or diaphragmatic recesses.
Recurrence may present with the same nonspecific symptoms as primary peritoneal spread: bloating, pain, ascites, and altered bowel habits. Rising carcinoembryonic antigen (CEA) levels or abnormal follow-up imaging often prompt reevaluation.
Management strategies for recurrence depend on the location, extent, and interval from initial treatment. Some patients may be candidates for repeat CRS and HIPEC, especially if the disease-free interval exceeds 12 months and recurrence is limited. Others may transition to systemic therapy or palliative care.
Close postoperative surveillance is crucial, with scheduled imaging and biomarker tracking. Each recurrence scenario must be re-evaluated by a multidisciplinary team to balance benefit, risk, and quality of life.
Role of Biomarkers in Surveillance and Decision-Making
Tumor markers such as CEA (carcinoembryonic antigen) and CA 19-9 are routinely used in follow-up. A rising CEA post-resection can be an early indicator of recurrence, though it lacks specificity. CA 19-9 may add value in mucinous tumors or when CEA is normal.
Emerging biomarkers include circulating tumor DNA (ctDNA), which may detect molecular relapse weeks to months before radiographic changes. These liquid biopsies offer a new frontier in surveillance and therapeutic stratification, particularly in patients under consideration for repeat surgery or systemic escalation.
Pathology-specific markers such as BRAF, KRAS, and MSI should be reassessed at recurrence if initial status was unknown or borderline. Tumor biology can evolve under treatment pressure, influencing response to chemotherapy and targeted therapies.
As with hormone receptor status in breast cancer or RET mutations in thyroid cancers, biomarker profiles now drive real-time therapeutic adjustments and recurrence risk models.
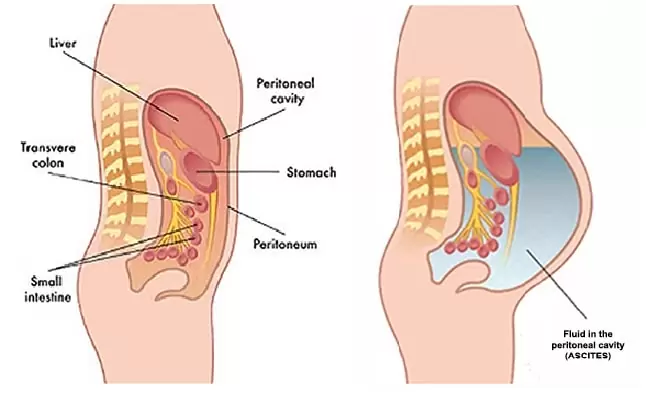
Nutritional Support and Symptom Management in Advanced Cases
Peritoneal metastasis often causes gastrointestinal dysfunction that severely impacts nutrition. Ascites, early satiety, nausea, and subclinical obstructions are common — all of which contribute to weight loss and muscle wasting (cachexia).
Nutritional assessment is essential from the time of diagnosis. In some cases, enteral feeding via nasogastric or jejunal tube may be required to support intake. Parenteral nutrition is considered when bowel function is compromised.
Symptom control remains a core goal in advanced disease. Diuretics may be used for ascites, antiemetics for nausea, and opioids for pain management. Intermittent paracentesis can relieve pressure and discomfort in refractory ascitic accumulation.
Palliative care integration at the time of peritoneal progression ensures patients receive comprehensive support for both physical and emotional symptoms, without prematurely withdrawing from oncologic therapy.
Palliative Strategies and Quality-of-Life Focus
When curative treatment is no longer feasible due to extensive peritoneal disease or poor performance status, the treatment focus shifts to palliation. This approach aims to manage symptoms, prolong meaningful survival, and preserve quality of life.
Key components include control of ascites, management of bowel obstruction, nutritional support, and pain relief. Peritoneal drainage catheters may be placed for recurring ascites. Venting gastrostomy tubes can provide relief from unmanageable gastric distension. Corticosteroids and prokinetics may reduce nausea and improve comfort.
Early integration of palliative care services has been shown to improve outcomes even when chemotherapy is continued. A coordinated team approach ensures that physical, psychological, social, and spiritual needs are addressed simultaneously — not as an afterthought, but as a critical component of comprehensive cancer care.
Differences Between Peritoneal Metastasis and Other Metastatic Patterns
Peritoneal spread behaves differently from hematogenous metastasis to organs like the liver or lungs. In liver metastasis, lesions are typically well-defined and can be targeted with local treatments such as ablation or embolization. In contrast, peritoneal carcinomatosis involves diffuse, scattered implants that encase and infiltrate soft tissues, leading to a complex pattern of disease.
Unlike other metastatic pathways, the peritoneal cavity lacks a centralized vascular drainage system, meaning that systemic chemotherapy may not reach tumor implants in high enough concentrations. This anatomic challenge is what makes HIPEC such a unique and valuable adjunct.
These anatomical and therapeutic differences mirror the diagnostic and clinical uniqueness seen in other cancer types. For example, the lack of a detectable mass in breast cancer without a lump similarly requires unique clinical awareness and treatment adaptation.
Multidisciplinary Approach and Center Specialization
Given the complexity of managing peritoneal metastasis from colon cancer, care should be centralized in high-volume centers with experience in both surgical and medical oncology. The best outcomes are achieved through multidisciplinary tumor boards, where surgeons, radiologists, oncologists, palliative specialists, nutritionists, and genetic counselors contribute to a unified care plan.
This team-based approach ensures proper selection for CRS+HIPEC, anticipates nutritional and functional challenges, and rapidly adapts therapy based on patient trajectory. Specialized centers also provide access to clinical trials, emerging biomarkers, and innovations such as immunotherapy or personalized ctDNA monitoring.
Patients benefit not just from technical expertise, but from coordinated, anticipatory care across specialties — the very model that defines modern precision oncology.
Early Recognition and Individualized Planning Matter
Colon cancer metastasis to the peritoneum represents a challenging and heterogeneous clinical entity. Yet, it is not hopeless. With improved imaging, molecular profiling, surgical techniques, and chemotherapy strategies, select patients can experience long-term survival — and even cure.
What matters most is early recognition, accurate staging, and prompt referral to specialized centers. Personalized treatment plans, informed by genetic and biologic markers, now allow for more targeted, effective, and patient-centered interventions than ever before.
Just as TULSA-PRO advanced the field of prostate cancer by localizing care with minimal damage, evolving strategies for peritoneal metastasis seek the same balance — effectiveness without overtreatment.
Frequently Asked Questions (FAQ)
What does it mean when colon cancer spreads to the peritoneum?
It means that cancer cells from the colon have migrated to the lining of the abdominal cavity, where they implant and grow as metastatic tumors. This form of metastasis is called peritoneal carcinomatosis and is considered stage IV disease, often requiring a specialized treatment approach.
How is peritoneal metastasis different from liver or lung metastasis?
Peritoneal metastases are often more diffuse and harder to detect or remove surgically. Unlike liver or lung lesions, they can encase organs and cause obstructive symptoms. They are also less responsive to standard systemic chemotherapy due to the peritoneum’s limited blood supply.
What symptoms might indicate peritoneal metastasis?
Common symptoms include abdominal bloating, persistent discomfort, nausea, vomiting, weight loss, and early satiety. These signs are typically caused by ascites, intestinal dysfunction, or peritoneal tumor growth affecting organ mobility.
Is it possible to detect peritoneal metastasis early?
Early detection is difficult due to vague symptoms and limitations in standard imaging. However, regular monitoring in high-risk patients and the use of sensitive tools like diagnostic laparoscopy or MRI can help detect smaller implants.
What is the Peritoneal Cancer Index (PCI)?
The PCI is a scoring system used to assess the extent of peritoneal metastases by evaluating 13 abdominal regions. Each region is scored based on tumor size, and the total score helps guide decisions on surgery and chemotherapy options.
What is HIPEC, and when is it used?
HIPEC (Hyperthermic Intraperitoneal Chemotherapy) is a heated chemotherapy treatment applied directly into the abdomen after surgical removal of visible tumors. It’s used in select patients with limited peritoneal disease and good functional status to reduce microscopic residual cancer cells.
Is CRS+HIPEC curative?
In selected cases with low PCI scores and no disease outside the abdomen, CRS+HIPEC offers a chance at long-term survival and even cure. However, it’s not appropriate for all patients and depends on careful preoperative evaluation.
Can peritoneal metastases recur after HIPEC?
Yes, recurrence is common. Even with optimal surgery and HIPEC, microscopic disease can remain. Recurrence is monitored using imaging, biomarkers like CEA, and sometimes repeat laparoscopy or surgery.
What systemic chemotherapy is used for peritoneal spread?
Typical regimens include 5-fluorouracil (5-FU), oxaliplatin, irinotecan, and targeted agents like bevacizumab. Therapy choice depends on tumor genetics (e.g., KRAS/BRAF mutation status) and prior treatment history.
Are genetic mutations relevant in peritoneal metastasis?
Yes. BRAF mutations and KRAS mutations are associated with higher rates of peritoneal spread and worse prognosis. Testing for these mutations helps guide treatment, including eligibility for targeted therapy or immunotherapy.
What role does CEA play in follow-up?
CEA is a tumor marker that often rises with recurrence. It is used to monitor response to treatment and detect early signs of disease progression, though it’s not definitive on its own and requires correlation with imaging.
How does nutrition impact treatment success?
Malnutrition is common due to gastrointestinal dysfunction. Addressing nutrition early — through diet adjustment, supplementation, or feeding tubes — improves treatment tolerance, immune function, and overall survival.
Can palliative care be used alongside active treatment?
Absolutely. Palliative care helps manage symptoms and improve quality of life without replacing oncologic care. Early integration is especially important in peritoneal metastasis due to the high symptom burden.
Are there any new research developments?
Yes. Ongoing trials explore immunotherapy, better HIPEC protocols, ctDNA for early relapse detection, and combinations of systemic and intraperitoneal therapies. These innovations aim to improve survival and personalize care.
How is this similar to other cancers that spread without forming a lump?
Like breast cancer without a lump, peritoneal metastasis can progress without classic findings. Both conditions require heightened awareness and reliance on imaging and biology, not just physical exam findings.