Can Titanium Implants Cause Cancer? What Science Reveals
- Foreword
- 1. Introduction
- 2. Understanding Titanium Implants
- 3. Biocompatibility of Titanium
- 4. Potential Risks and Complications
- 5. Titanium Implants and Cancer: Evaluating the Evidence
- 6. Factors Influencing Cancer Risk
- 7. Alternative Materials and Technologies
- 10. Frequently Asked Questions (FAQs)
- Closing Thoughts
Introduction
If you’ve landed here with a slightly raised eyebrow over your titanium implant — or you’re considering one and doing your due diligence — you’re not alone. The question, “Can titanium implants cause cancer?” is more common than you might think. And it’s not the stuff of wild speculation or internet conspiracy. It taps into a very real, very human concern: how can we be sure that the materials placed inside our bodies for years — even decades — are truly safe?
Titanium implants have become a mainstay in modern medicine and dentistry. They’re in our hips, knees, dental arches, and even our spines. Strong, lightweight, biocompatible — titanium has long been regarded as the golden child of implant materials. But does that reputation still hold up under scrutiny?
Let’s get to the heart of it: Is there a cancer risk? Should you be worried if you already have a titanium plate or dental screw quietly sitting beneath your skin or jawbone?
The short answer is: the scientific consensus currently leans heavily toward “no direct evidence of causation.” But — and it’s an important “but” — as with most things in medicine, the real picture is more nuanced. That’s why this article exists: to walk you through not just what we know, but how we know it, what questions remain, and what kind of logic and evidence have shaped our current understanding.
Before we dive into studies, mechanisms, materials, and expert opinions, it’s important to frame the discussion correctly. The idea that implants — especially metallic ones — might have long-term biological effects isn’t outlandish. After all, your body is not a passive container. It’s a dynamic, chemically active system. And when you introduce foreign material, no matter how ‘inert’ it may seem, some level of interaction is inevitable.
Let’s also be honest with ourselves: the public’s trust in medical devices has been rattled before. From problematic hip replacements to recalled breast implants, skepticism isn’t paranoia — it’s a form of self-preservation. So if you’re asking these questions, you’re not being alarmist. You’re being thorough.
So here’s the promise of this article: no fear-mongering, no downplaying. We’ll unpack the scientific literature, challenge assumptions, explore alternative materials, and bring in perspectives from both research and clinical practice.
This debate isn’t limited to titanium. If you’re comparing implant materials, check out what we found on dental crowns and cancer safety.
Whether you’re a patient, a caregiver, a health professional, or just an intensely curious person, you deserve not just to be reassured — but to be informed. And that’s what we’re going to do.
Ready to look deeper beneath the surface — quite literally? Let’s start with the metal itself: titanium. Why has it been trusted for so long, and what exactly is going on at the molecular level when it becomes part of you?
Onward.
Understanding Titanium Implants
Before we can examine the risk, we need to understand the material itself. What makes titanium so popular in medicine? Why has it become the go-to metal for something as intimate and high-stakes as a bodily implant?
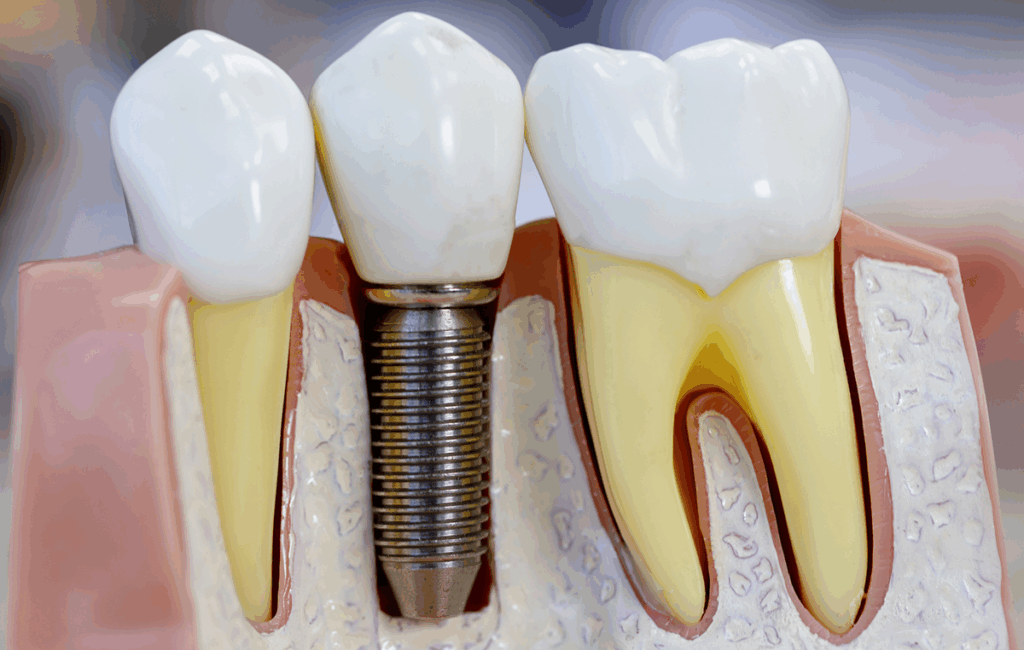
Let’s rewind the clock a bit. Titanium wasn’t always the biomaterial of choice. In fact, its story as a medical marvel didn’t take off until the mid-20th century, when a Swedish orthopedic surgeon named Per-Ingvar Brånemark made a serendipitous discovery: titanium screws inserted into bone could become almost impossible to remove — not because of scarring or encapsulation, but because the bone actually bonded with the metal. This process was dubbed osseointegration, and it changed the game.
What Are Titanium Implants?
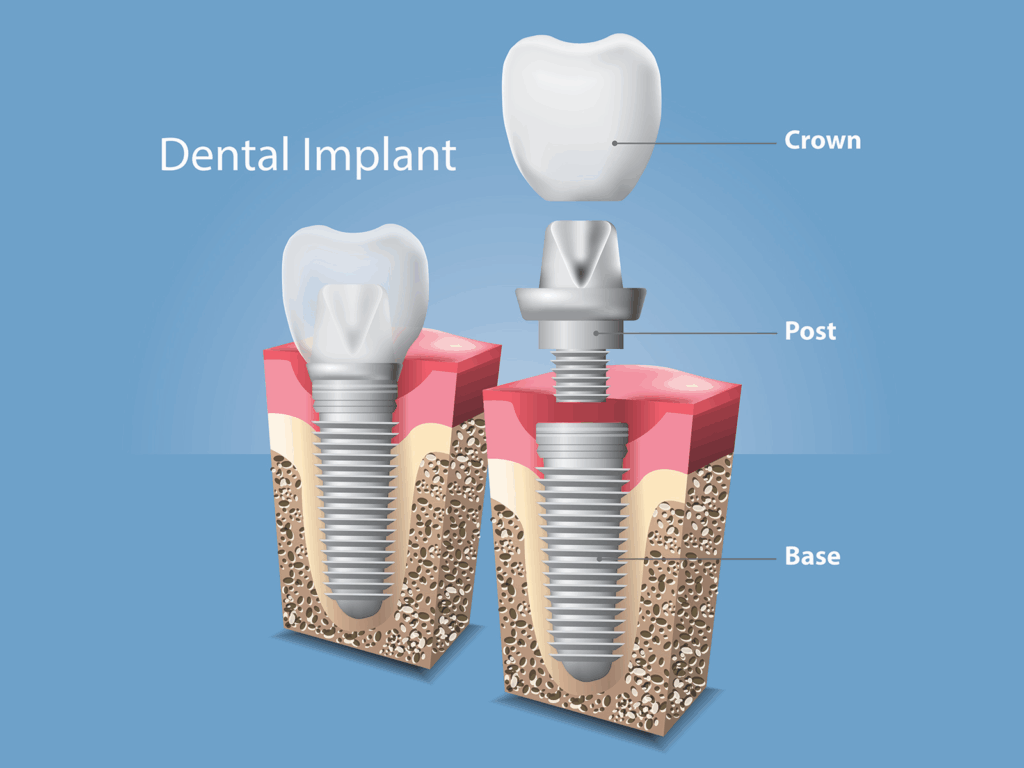
In the simplest terms, titanium implants are medical devices made from titanium or titanium alloys that are placed inside the body to replace or support biological structures. Think hip and knee replacements, dental implants, spinal rods, cranial plates — even some pacemaker casings.
But these aren’t just hunks of metal. They’re precisely engineered, sterilized, and often surface-treated devices intended to function in harmony with your body’s tissues. The idea isn’t just to “stick” something in — it’s to make it stay, stay quietly, and ideally stay forever.
Why Titanium?
Why not stainless steel, or cobalt-chrome, or some fancy space-age ceramic? What makes titanium so special?
Start with the basics: titanium is strong but lightweight, with an exceptional strength-to-weight ratio. For a hip joint, that means the implant can handle the force of your stride without adding unnecessary load.

Then there’s corrosion resistance. Your body is essentially a warm, salty, moist environment — perfect conditions for rust and degradation. Titanium, thanks to its ability to form a thin but incredibly stable oxide layer on its surface, resists that kind of chemical breakdown. It doesn’t corrode like steel or leach like some other metals.
But perhaps the most critical quality is biocompatibility. Titanium is remarkably non-reactive with biological tissues. The immune system, usually quick to attack anything foreign, tends to tolerate titanium. It’s not that your body ignores it — it just doesn’t freak out about it.
And if we’re being precise, most medical-grade implants aren’t pure titanium, but titanium alloys — often mixed with small amounts of aluminum and vanadium (you’ll see this labeled as Ti-6Al-4V, which is aerospace-grade titanium). These tweaks enhance mechanical properties, like flexibility and fatigue resistance, without compromising too much on biocompatibility. Or so we’ve thought — we’ll revisit that alloying question later when we talk about risk factors.
Still, you might wonder: if titanium is so perfect, why is anyone worried about it causing cancer?
That’s a good question. The short answer is: nothing placed in the body is entirely inert. Titanium particles can shed over time through mechanical wear or corrosion, especially at the microscopic level. These particles — and the body’s response to them — are part of the conversation we’ll have in more depth later.
Types of Titanium Implants
Let’s pause for a moment to appreciate how versatile this metal really is. When we talk about “titanium implants,” we’re casting a wide net:
- Orthopedic implants: Think of plates and screws used in fracture fixation, joint replacements, and spinal fusion rods. These are often large and load-bearing.
- Dental implants: These are probably the most well-known, involving small titanium posts surgically embedded in the jawbone to anchor prosthetic teeth.
- Craniofacial implants: Used in reconstructive surgery, especially after trauma or tumor removal.
- Cardiovascular devices: Certain stents, pacemaker casings, and vascular clips may use titanium components due to their strength and corrosion resistance.
And here’s the thing — not all implants are created equal. The risks, including theoretical cancer risk, may differ between a massive hip prosthesis and a tiny dental screw, depending on factors like surface area, mechanical load, and whether the implant is exposed to body fluids or soft tissue over time.
One more thought before we move on: sometimes, people think that if they don’t “feel” the implant, it must be totally inactive. But that’s not quite true. Your body and the implant are in constant, low-level biochemical dialogue. Cells interact with the surface. Immune responses can be activated, even subtly. Over years, mechanical stress and chemical exposure can lead to tiny byproducts — metal ions, microscopic wear particles — entering local tissues or the bloodstream. And it’s these processes, not the bulk implant itself, that form the basis of concern when we ask about cancer.
So now that we understand what titanium implants are and why they’re used, let’s look at what the body actually does with them. What happens once titanium becomes part of you — and why does that matter for your long-term health?
Next up: the biological dance known as biocompatibility.
Biocompatibility of Titanium
Let’s move past the structural marvel that is titanium and into the living, breathing context of the human body — because that’s where things get interesting.
You might assume that if a material doesn’t cause a violent allergic reaction or obvious rejection, it must be “biocompatible.” In reality, biocompatibility isn’t a binary yes/no. It’s a spectrum — a relationship. And like any relationship, how well it works depends on the circumstances, the personalities involved (in this case, your cells and the titanium surface), and time.
So, what happens when titanium enters your body and sets up camp?
Osseointegration: The Gold Standard
If you’ve ever wondered how a dental implant doesn’t just wobble around or fall out like a loose screw, the answer is osseointegration. This is the cornerstone of titanium’s appeal.
Osseointegration isn’t just titanium being tolerated — it’s bone growing into it. That’s an astonishing concept if you pause to consider it. Bone cells (osteoblasts) literally adhere to the titanium’s surface, colonize it, and lay down new bone as if it were a natural part of the skeletal system. No scar tissue, no encapsulating membrane — just seamless integration.
This doesn’t happen with most other materials. Titanium’s unique surface chemistry, especially the thin oxide layer that forms spontaneously in air or body fluids, seems to encourage this biologically friendly embrace. In effect, the body is not just permitting titanium to exist; it’s actively incorporating it into its own structure.
But again, this isn’t magic. Osseointegration works best under specific conditions: stable mechanical positioning, proper loading, minimal infection, and a host of patient-specific variables like bone density, systemic health, and even genetics.
There’s also a natural crossover here with oral pathology — oral cancer progression and symptoms help frame the conversation around what’s actually likely.
So when it goes right, it’s spectacular. When it goes wrong — infection, loosening, or non-integration — the entire notion of biocompatibility gets called into question. And this opens the door to a more nuanced risk landscape.
Corrosion Resistance: Silent but Critical
You don’t see titanium implants rusting or pitting the way you might with cheaper metals. Why? The secret lies in the oxide layer — a passive film of titanium dioxide that forms the instant titanium touches oxygen. This layer is ultrathin (we’re talking nanometers), but it’s fiercely protective. It repels chemical attacks from body fluids, enzymes, and the immune system. It also helps minimize ion release, which is critical when we’re talking about potential systemic effects like inflammation or, yes, cancer.
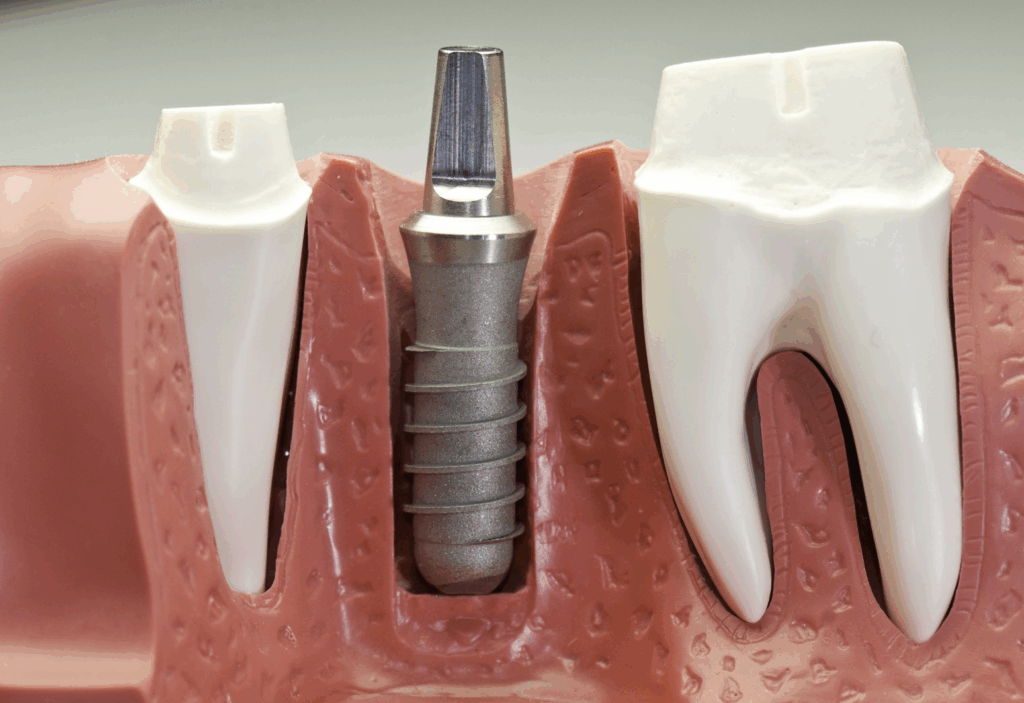
But here’s the twist: that oxide layer can be damaged. Repeated micromotions, friction between implant parts, or exposure to a particularly hostile biochemical environment (such as in chronic inflammation or in smokers) can wear down that barrier. When that happens, titanium ions or nanoparticles may begin to leach into surrounding tissue — and potentially into the bloodstream.
This isn’t fear-mongering — it’s reality. Wear particles and ions have been detected in the tissues around implants and in remote organs like the liver and spleen. Does this automatically mean cancer? No. But it adds complexity to what we mean when we say “safe.”
Immune Response: The Body Has Opinions
Here’s where the conversation becomes even more interesting. Just because the body doesn’t launch a full-scale immune assault doesn’t mean it’s indifferent to the presence of titanium. Immune surveillance is always active, and titanium does provoke a subtle, often low-grade response.
In most people, this response is minimal and well-managed. Macrophages (the cleanup crew of the immune system) may accumulate around the implant site and deal with any wear particles that show up. But in some cases — particularly when particles are small enough to be taken up by cells — this can lead to a state known as chronic inflammation.
And chronic inflammation is, as we now know, a risk factor for several long-term pathologies, including carcinogenesis. That doesn’t mean titanium causes cancer directly, but it does raise the bar for evaluating how titanium interacts with the immune system over time.
Moreover, some individuals may have hypersensitivity reactions to titanium or its alloys. These are rare, but real. Symptoms can range from dermatitis and joint pain to fatigue or local inflammation that just doesn’t resolve. These aren’t signs of overt rejection, but they suggest a more nuanced immunological dance — one that varies significantly from person to person.
And let’s not forget the alloying elements. Titanium itself may be relatively inert, but what about the aluminum or vanadium added for strength? These metals have their own profiles, and their ion release — however minuscule — may trigger different biological responses. That’s something often left out of the “titanium is safe” narrative.
So What’s the Verdict So Far?
Titanium is extraordinarily well tolerated by the human body — more than most metals. But it’s not invisible to biology. It’s more like a well-mannered guest at the cellular dinner table: generally polite, occasionally messy, and, in rare cases, a source of long-term tension.
For the vast majority of patients, the biocompatibility of titanium is a triumph of medical science. But it’s not invincible. And it’s this middle ground — this acknowledgment of titanium’s strengths and its small, but real, vulnerabilities — that lays the groundwork for a meaningful discussion about risk.
Now that we’ve explored how titanium behaves in the body under “normal” circumstances, we’re ready to confront the central question: Can it go wrong in a way that leads to cancer? That’s where we’re headed next — into the risk territory, with our scientific lens sharpened and our assumptions left at the door.
Potential Risks and Complications
By now, we’ve established that titanium is, in many ways, a remarkable material. But even remarkable materials can come with caveats. And in the context of implants, “biocompatible” doesn’t mean “risk-free.” It means the risks are managed — ideally minimized, but never eliminated entirely.
So let’s step into the murkier waters. Because while cancer might be the headline concern that brings people here, it rarely exists in a vacuum. Before we can meaningfully weigh the cancer question, we need to look at the broader landscape of complications — especially the ones that could lay the groundwork for something more serious.
General Complications: The Usual Suspects
Every implant procedure — titanium or otherwise — carries inherent risks. That’s not fear-mongering; that’s surgical reality.
Infection is probably the most familiar. Even in sterile operating environments, bacteria can sneak in. Once they find a home on the surface of an implant — especially if that surface is slightly textured or roughened to encourage bone growth — they can be maddeningly difficult to dislodge. Unlike living tissue, metal doesn’t mount an immune defense. It becomes a kind of bacterial safe house, and sometimes the only way to clear the infection is to remove the implant altogether.
Then there’s mechanical failure. Over time, implants can loosen, fracture, or migrate. This isn’t just a mechanical issue — it has biological consequences. Movement between an implant and surrounding tissue generates wear particles, which we’ll return to shortly, and can provoke localized inflammation. If that inflammation becomes chronic, the body may start to dissolve nearby bone in a process known as osteolysis — a major cause of implant failure.
But let’s not lose the forest for the trees. These are complications most patients never experience. When they do occur, they’re usually caught and managed. But they represent the baseline risks — the soil in which other, rarer complications can take root.
Allergic Reactions: Rare, But Not Imaginary
It surprises many patients to learn that you can have an allergic reaction to titanium. The myth of total inertness persists, likely because allergic responses are so uncommon. But uncommon doesn’t mean impossible.
Most cases involve type IV hypersensitivity, a delayed form of immune response. It doesn’t show up as anaphylaxis or rash in the way nickel allergies might. Instead, symptoms are more insidious: persistent inflammation around the implant, unexplained pain, implant loosening without infection, or systemic symptoms like fatigue and brain fog. For those affected, the experience can be maddening — and difficult to diagnose.
Part of the problem is that standard allergy tests (like skin patch testing) aren’t very reliable for metals like titanium. Some specialists use more advanced assays, like MELISA testing, which evaluates immune cell reactivity to specific metal ions. But this kind of testing isn’t widely available, nor is it universally accepted.
Even when a hypersensitivity is confirmed, management options are limited. Replacement with an alternative material — usually zirconia — may be necessary, but that brings its own risks and tradeoffs.
And just to add another layer: reactions may not be to titanium itself, but to the alloying elements. Remember that Ti-6Al-4V we mentioned earlier? That vanadium and aluminum could, in some cases, be the real immunological irritants. It’s another reminder that “titanium” isn’t always pure titanium.
Metallosis: A Hidden Threat
Now we arrive at a lesser-known but increasingly relevant complication: metallosis. It’s a term that’s gotten more attention in orthopedic circles, particularly in relation to metal-on-metal hip replacements. But it can occur with any implant that sheds metal debris into surrounding tissues.
When microscopic particles — or ions — from titanium or its alloys accumulate in soft tissue, the body doesn’t exactly thank you. Macrophages engulf the particles, triggering the release of inflammatory cytokines. Over time, this can lead to discoloration of the surrounding tissue (often gray or black), pain, swelling, and — critically — bone loss. That’s a local problem.
But the concern doesn’t end there. There’s evidence that these particles can enter systemic circulation and be deposited in distant organs like the liver, spleen, and even the brain. The clinical significance of this systemic distribution remains poorly understood, but it raises obvious questions. What happens when these particles persist in tissue for years or decades? Do they accumulate? Are they cleared? Do they alter local cellular behavior?
Now, to be fair: metallosis is rare with titanium compared to other metals. But rare doesn’t mean irrelevant. Especially not if you’re one of the patients dealing with persistent pain and no clear diagnosis — until someone thinks to biopsy the tissue around your implant and finds dark-staining macrophages packed with titanium debris.
And while metallosis itself isn’t cancer, it creates a chronic inflammatory microenvironment — and that, as we’ll soon explore, can be a factor in cellular dysregulation and possibly carcinogenesis.
Putting It Together: Why These Risks Matter
All of these complications — infection, hypersensitivity, metallosis — are not just stand-alone problems. They intersect. A patient with undiagnosed titanium allergy may have a higher risk of implant failure. A failing implant may shed more particles. Those particles may inflame tissue. And chronically inflamed tissue, under the right (or wrong) conditions, may undergo cellular changes that — over years — elevate cancer risk.
This isn’t inevitable. In fact, it’s exceedingly rare. But this chain of events is biologically plausible, and it’s part of why the question “can titanium implants cause cancer?” isn’t as easily dismissed as some might think.
Now that we’ve laid out the playing field — the known complications, the biological interactions, the rare but real consequences — we’re finally ready to tackle the big question head-on. Not in theory, not in speculation, but in data.
Next, we’ll pull the research into focus and ask: what does the evidence actually say about titanium and cancer? Let’s get into it.
Titanium Implants and Cancer: Evaluating the Evidence
Now we come to the question that brought us here in the first place. It’s the one that tends to simmer in the back of the mind, especially late at night when you’ve just read an article with the words “metal ions,” “chronic inflammation,” and “systemic toxicity” all in the same paragraph.
Can titanium implants cause cancer?
This isn’t a question medicine takes lightly — nor should it. When you place a foreign material inside the body for years, or decades, or even a lifetime, it’s not just about whether it performs its mechanical job. It’s about whether it does so without triggering unintended and potentially catastrophic biological side effects. Cancer, by its very nature, is a disease of biological miscommunication — uncontrolled cellular growth born from stress, mutation, or dysfunction in cellular signaling. So the concern that a long-term implant might, under certain conditions, contribute to such dysfunction isn’t an outlandish one.
But let’s be rigorous: what does the evidence actually show?
Overview of Research
The good news, right up front, is that there is no conclusive evidence linking titanium implants to an increased risk of cancer in the general population. Not in humans, not in animals — at least not under normal conditions.
A large body of epidemiological data, especially from decades of use in dental and orthopedic applications, simply doesn’t show a statistically significant uptick in cancer diagnoses among implant recipients. This is worth emphasizing: with millions of implants placed worldwide over many years, if titanium had a strong carcinogenic signal, we’d likely have seen it by now.
But — and this is where nuance matters — “absence of evidence is not evidence of absence.” The bulk of our data focuses on short-to-intermediate term outcomes (5–15 years), and often the sample sizes are diluted by the heterogeneity of patients and implant types. Moreover, subtle or delayed effects — like tissue-level changes that might contribute to rare cancers over decades — are harder to detect in broad cohort studies.
Case Studies: The Outliers That Demand Attention
Scattered throughout the literature are isolated case reports that do raise eyebrows. A handful of studies describe sarcomas — typically aggressive cancers of connective tissue — developing near titanium implants. These are exceedingly rare, and causality is almost impossible to establish. After all, correlation does not imply causation. Could it be coincidence? Of course. Could it be a localized response to chronic inflammation or particle debris in a genetically susceptible individual? That’s also plausible.
Some of these cases involve orthopedic implants placed after traumatic injury or in patients with pre-existing risk factors. The cancer often arises years after implantation, which muddies the timeline even further. Importantly, similar cases have been reported with implants made from other materials too, including stainless steel and cobalt-chrome alloys — suggesting that any foreign body, under certain rare conditions, might contribute to a carcinogenic microenvironment.
The mechanism? Chronic irritation, persistent inflammation, oxidative stress, and — in some studies — the potential genotoxicity of metal ions at high enough concentrations. But again, these conditions are exceptional, not typical. It’s not the titanium per se that’s carcinogenic, but what it becomes in a worst-case biological scenario.
Regulatory and Scientific Consensus
What do health authorities say? As of this writing, titanium and its commonly used alloys are not classified as carcinogenic to humans by the International Agency for Research on Cancer (IARC), the World Health Organization’s leading authority on the matter. Nor have agencies like the FDA or European Medicines Agency flagged titanium as a cancer risk in the context of medical implants.
This doesn’t mean regulators aren’t watching. It just means the data so far doesn’t warrant alarm. Titanium is one of the most studied metals in biomaterials science. It’s used in everything from orthopedic screws to heart valve housings, from spinal cages to dental roots — and this extensive track record plays a significant role in its regulatory standing.
That said, researchers are not resting on historical reassurance. There’s growing interest in nano-scale interactions, particle toxicity, and inflammation-mediated carcinogenesis. Animal studies have shown that if titanium nanoparticles are injected in high concentrations or administered under unusual circumstances, they can produce DNA damage in cells. Whether this translates to cancer risk in humans under real-world implant conditions is still a subject of ongoing research — and rightly so.
So where does this leave us?
It would be dishonest to claim there is zero risk, in the most absolute sense. There is no such thing as zero risk in medicine, especially when we’re dealing with long-term foreign-body implantation. But there’s also no compelling, reproducible, large-scale evidence that titanium implants cause cancer under normal clinical use.
What we can say is this: cancer is a complex, multifactorial disease. Titanium implants are not carcinogens in the classical sense, like tobacco smoke or asbestos. But like any foreign material, under rare and specific conditions — such as in patients with chronic inflammation, hypersensitivity, or heavy metal ion exposure — they may be one factor in a much larger puzzle.
And that, again, is why intelligent scrutiny matters. Because even if the absolute risk is small, the biological plausibilityinvites us to stay vigilant — not afraid, but informed.
Next, we’ll explore what might make some people more vulnerable than others. Because if titanium alone isn’t the villain, maybe the story is really about context — the patient, the surgical technique, and the long-term environment around the implant. That’s what we’ll dive into next.
Factors Influencing Cancer Risk
If titanium implants don’t directly cause cancer, why does the question persist? Partly, it’s human nature — we look for explanations when things go wrong. But partly, it’s because risk is rarely a single-thread issue. It’s a fabric woven from multiple factors, and when we’re talking about cancer — a disease of cumulative biological insult — context is everything.
So let’s shift the lens. Rather than asking, “Can titanium cause cancer?”, it may be more illuminating to ask, “What conditions might make cancer more likely in the presence of a titanium implant?” Because here’s the truth: the risk — if it exists — likely hinges on what is implanted, who it’s implanted into, and how the body responds over time.
Implant Material Composition
Not all titanium is created equal. The name itself can be a bit misleading. Most medical implants aren’t made from pure titanium, but from titanium alloys — primarily Ti-6Al-4V, a blend of titanium, aluminum, and vanadium. The alloy is prized for its mechanical resilience, but its biological footprint is less straightforward.
Why does this matter?
Because while titanium itself is highly corrosion-resistant and generally well-tolerated, the alloying elements may behave differently. Aluminum and vanadium ions, in particular, have raised eyebrows in the toxicology literature. In certain in vitro studies, these ions have shown genotoxic effects — meaning they can damage DNA under specific conditions.
Now, before panic sets in: the levels of ion release from standard implants are extremely low under normal wear. But in the presence of mechanical stress, micromotion, or corrosion, tiny quantities of these ions can be released into surrounding tissues or enter systemic circulation. The biological effect of chronic, low-level exposure over decades is still being studied. It’s likely to be negligible for most people — but perhaps not for all.
And if you’re exploring diagnostics, what dental X-rays can reveal might help clarify where implants fit into that big picture.
Surface treatments and coatings add another layer of complexity. Implants are often treated to encourage bone bonding or resist bacterial colonization. These coatings (such as hydroxyapatite, plasma-sprayed titanium, or even silver-ion layers) can alter how the implant interacts with tissue, including how particles are released when wear occurs. Each variable changes the equation.
Patient-Specific Factors
This is where things get even more nuanced. Two people can receive identical implants in the same anatomical location and have completely different outcomes. Why?
Because biology isn’t uniform. It’s personal.
Take genetics, for starters. Some individuals have mutations in genes involved in DNA repair, oxidative stress response, or immune regulation. In these patients, any additional biological burden — whether from inflammation, ion exposure, or chronic irritation — may push cellular processes closer to dysregulation. Add an implant to the mix, and you’ve got a different risk landscape than the average patient.
Immune reactivity is another variable. Most people’s immune systems barely acknowledge a titanium implant after healing. But some individuals, due to metal sensitivity or autoimmune tendencies, experience persistent low-level inflammation at the implant site. This doesn’t always result in obvious symptoms — but at a tissue level, it can mean a pro-inflammatory environment that might, over years, shift cellular behavior toward instability.
And then there are lifestyle factors. Smoking, poor nutrition, chronic illness (especially diabetes or autoimmune disease), and environmental toxin exposure can all impair the body’s ability to manage stress at the cellular level. When these factors intersect with the presence of a long-term implant, the overall burden increases.
Even age plays a role. Younger patients may have decades of exposure ahead of them, meaning more time for complications (however rare) to develop. Older patients may have diminished cellular repair mechanisms, altering their baseline cancer risk in different ways.
It’s a reminder that medicine isn’t binary. It’s probabilistic — built on likelihoods, not absolutes.
Surgical Technique and Postoperative Care
It’s easy to overlook the human factor — the surgeon’s hand, the patient’s recovery, the quality of follow-up care. But these elements can dramatically influence the biological environment around an implant.
For example, an implant that is improperly seated, moves over time, or causes micromotion can lead to fretting corrosion — a form of localized wear that generates tiny metal particles. These particles don’t just disappear. They accumulate in the surrounding tissues and may eventually enter systemic circulation. Their presence can provoke inflammation, recruit immune cells, and — in rare cases — alter cellular behavior.
Similarly, infection is a critical variable. An infected implant site becomes a hotbed of immune activity, cytokine release, and tissue turnover. That chronic inflammatory environment, even when subclinical, is a well-established contributor to carcinogenesis. Again, we’re not saying infection leads to cancer. We’re saying chronic, unresolved inflammation might, and implants can, under the wrong conditions, create that environment.
Then there’s postoperative surveillance. Patients who skip follow-up appointments or dismiss lingering symptoms may miss early signs of trouble. Early intervention — whether for loosening, infection, or unexplained inflammation — can prevent years of silent biological stress that might otherwise go unchecked.
Surgical technique and follow-up care don’t just determine whether an implant succeeds mechanically. They help shape the microenvironment that either keeps biology in equilibrium — or tilts it toward pathology.
So what are we really saying here?
The risk of cancer from titanium implants isn’t a fixed property of the material. It’s an emergent property of a system — one that includes the metal, the biology it encounters, and the context in which it lives. For most patients, that system remains stable and benign. But in certain corners of that matrix — genetically predisposed individuals, patients with chronic inflammation, implants that shed more particles than expected — the equilibrium may shift.
Does that mean titanium implants are unsafe? No. It means they’re part of a complex equation — and like any medical device, they must be chosen and managed with a full view of that complexity.
In the next section, we’ll look at what alternatives exist — and whether there are materials or technologies that might reduce even these already rare risks. Because for some patients, it’s not just about reassurance. It’s about options.
Alternative Materials and Technologies
So far, we’ve painted titanium as a mostly reliable, occasionally finicky partner in the world of implants — not dangerous by nature, but not immune to complications either. And for most people, titanium will continue to be the material of choice. But what if you’re in that sliver of the population who doesn’t tolerate it well? Or what if you’re simply the kind of person who wants every option on the table before consenting to a lifelong relationship with a metal device inside your body?

This is where the conversation opens up. Because while titanium dominates the scene, it’s not alone. Other materials — some older, some cutting-edge — offer compelling alternatives, each with their own strengths, drawbacks, and clinical nuances.
Zirconia Implants: The Ceramic Challenger
Ask any biological dentist or implantologist about alternatives to titanium, and the first word out of their mouth is usually “zirconia.”
Zirconia implants — made from yttria-stabilized zirconium dioxide — are not metal in the traditional sense. They’re ceramic. And while “ceramic” might conjure images of fragile coffee mugs, zirconia is anything but brittle. It’s tough, hard, and incredibly resistant to corrosion — all the more reason it’s gaining traction, especially in dental applications.
Why the growing interest?
For one, zirconia is often marketed as “metal-free,” a claim that appeals to patients with metal sensitivities or those wary of long-term exposure to metallic ions. It also has excellent biocompatibility. Early studies show low inflammatory response, minimal plaque adhesion, and good soft tissue integration — all of which make it particularly attractive in the mouth, where aesthetics and hygiene matter.
But zirconia isn’t without its limitations. It doesn’t integrate with bone quite as predictably as titanium, and it’s less forgiving under mechanical stress. One good knock in the wrong direction, and it’s more likely to fracture than bend — which is a key difference in clinical risk profile. Surgical placement is also more technique-sensitive, leaving less room for error.
Still, for patients with metal allergies or aesthetic concerns (zirconia is white, which means no graying at the gumline), it’s a serious contender — especially in single-tooth implants where load stress is manageable.
Emerging Materials: Beyond the Familiar
Step outside the mainstream, and you’ll find a growing body of research exploring entirely new classes of implant materials — many of them designed at the molecular level to optimize specific traits: antibacterial properties, controlled degradation, enhanced bone bonding, and yes, reduced long-term toxicity.
One such class is bioactive glasses — silica-based materials that can stimulate bone growth and even dissolve over time, making them ideal for certain temporary implants or coatings.
Then there are PEEK-based implants (polyether ether ketone), which are polymers rather than metals. PEEK is radiolucent (doesn’t show up on X-rays), lightweight, and has a modulus of elasticity closer to bone than titanium — which can reduce stress shielding. It’s increasingly used in spinal surgeries and orthopedics, especially when MRI compatibility is important. However, pure PEEK isn’t osteoconductive, meaning it doesn’t naturally fuse with bone, so it’s often coated with titanium or hydroxyapatite to improve integration — and that brings titanium back into the equation.
On the far horizon, researchers are exploring graphene-based composites, magnesium alloys (which are biodegradable and ideal for temporary use), and even 3D-printed hybrid materials that combine the best of metal and polymer science. These are still in early stages, but the direction is clear: implants of the future may be biologically active, custom-shaped, and far more intelligent than anything we use today.
Advances in Implant Design: Smarter, Safer, More Selective
While the composition of the material gets a lot of attention, design matters just as much — maybe more. How an implant is shaped, textured, and positioned can dramatically affect how your body responds to it.
Take surface engineering. Titanium implants today are often nano-textured to enhance cell attachment and speed up osseointegration. Some are even coated with bioactive molecules — proteins, peptides, or antimicrobial agents — designed to coax specific biological behaviors. The goal is no longer just to avoid rejection; it’s to actively shape the healing response.
We’re also seeing advances in modular implant systems, especially in orthopedics, where the load-bearing components can be swapped out or upgraded without replacing the entire structure. This minimizes surgical trauma during revision procedures and extends the lifespan of the original implant.
And let’s not forget the role of digital planning and custom-fit implants, especially with the rise of high-resolution 3D imaging. Surgeons can now create patient-specific guides, simulate outcomes before the first incision, and even 3D-print implants tailored to a person’s unique anatomy. This level of personalization not only improves mechanical fit — it reduces micromotion, which, as we’ve seen, is one of the major drivers of particle wear and inflammation.
In other words, safety isn’t just a function of material. It’s a synergy of material, design, placement, and biology — and that synergy is getting smarter by the year.
In stepping away from titanium, you’re not walking into a void. You’re walking into a landscape of evolving options, many of which are grounded in serious science and clinical experience.
Of course, no alternative is perfect. Each comes with its own tradeoffs — mechanical, biological, financial. And in many cases, titanium remains the better choice. But for certain patients, whether due to metal sensitivity, prior complications, or long-term risk considerations, these alternatives aren’t just “nice to know.” They’re essential.
In our next section, we’ll bring in the voices of professionals who live and breathe these decisions. Because while we’ve been unpacking the data, there’s a lot to learn from what experienced surgeons and researchers actually see in practice. Let’s hear what they have to say.
Frequently Asked Questions (FAQs)
1. Can titanium implants cause cancer?
Let’s get straight to the heart of the matter. The vast majority of research — across decades, disciplines, and global populations — has not found a direct causal link between titanium implants and cancer. That’s a meaningful point of reassurance.
But that doesn’t mean the conversation ends there. In medicine, very few things are black and white. While titanium itself isn’t classified as a carcinogen, some of the rare complications associated with it — like chronic inflammation, immune dysregulation, or particle shedding — can, under certain circumstances, create a tissue environment more vulnerable to cellular mutation. These are edge cases, not the rule, but they warrant honest attention.
So, can titanium “cause” cancer? Not in the simple, direct way that smoking causes lung cancer or UV rays contribute to skin cancer. But under specific biological conditions, in sensitive individuals, and after long periods of stress, it could play a role — likely a small one, and almost always as a co-factor rather than a trigger.
2. What are the symptoms of a negative reaction to titanium?
Negative reactions don’t always announce themselves clearly. Some patients experience persistent discomfort at the implant site, localized inflammation, unexplained pain, or even implant loosening despite clean scans and normal bloodwork.
Others report systemic symptoms: chronic fatigue, diffuse joint pain, rashes, or a general sense that “something’s off” following implant surgery. These symptoms are non-specific and overlap with dozens of other conditions, which makes diagnosis challenging. But when they coincide with the timeline of implantation and no other cause is found, the implant becomes a logical place to look.
The key here is pattern recognition — not jumping to conclusions, but also not dismissing persistent symptoms that don’t respond to conventional treatment.
3. Are there safer alternatives to titanium?
“Safer” is context-dependent. For most people, titanium is both safe and effective. But for patients with documented metal allergies, unexplained immune reactivity, or a personal preference to avoid metal altogether, zirconia implants are emerging as a viable alternative — particularly in dentistry.
Other materials like PEEK (a high-performance polymer) and magnesium-based implants are being explored in orthopedic and spinal surgery, though they come with their own trade-offs in terms of strength, longevity, and integration.
No material is perfect. But yes — there are alternatives, and the best choice depends on your medical history, the type of implant, and your long-term health goals.
4. How can I minimize risks associated with titanium implants?
Start by choosing a highly experienced surgeon who uses evidence-based practices and pays attention to detail — not just in surgical technique, but in material selection, implant positioning, and patient-specific planning.
If you have a history of allergies, autoimmune disorders, or unexplained inflammatory conditions, talk to your doctor about pre-operative metal sensitivity testing — even if it’s not standard protocol. It might give both of you more clarity.
And post-op, don’t ghost your care team. Follow-up matters. Early signs of trouble are often subtle: a slight shift in how the implant feels, minor swelling that doesn’t resolve, or fatigue that starts to creep in. Document everything, and advocate for yourself if something feels wrong — even if the tests look “normal.”
Your job isn’t to worry constantly; it’s to stay connected to your own baseline. When that changes, speak up.
5. Should I get tested for metal allergies before receiving an implant?
If you’ve had allergic reactions to metal jewelry, dental appliances, or previous implants, the answer is yes — you should absolutely bring it up and consider testing. Not all tests are equally informative, but advanced assays like MELISA can detect immune cell responses to specific metals, including titanium, vanadium, and nickel.
For the general population with no history of metal sensitivity, routine allergy testing isn’t typically recommended. But if there’s any doubt — especially if you’re prone to autoimmune issues or chronic inflammation — having the conversation is worth your time. It’s a small step that can help avoid a potentially long and frustrating road later.
These questions often serve as jumping-off points for deeper discussions. And that’s the real takeaway: when it comes to implants — titanium or otherwise — the smartest thing you can do is stay curious, stay informed, and keep your medical care collaborative.
You’re not a passive recipient of a product; you’re the biological environment it depends on to succeed. That makes your perspective central, not peripheral — and your questions, no matter how small they seem, always worth asking.
In our final section, we’ll wrap it all up with a clear-eyed reflection on what this all means for you, your health, and the evolving future of implant medicine.
Closing Thoughts
By now, you’ve likely gathered that the story of titanium implants and cancer isn’t a neat one. It isn’t reducible to headlines or binary answers. There is no definitive “yes” or “no,” and that’s not a flaw in the science — it’s a reflection of the complexity of human biology, the diversity of individual immune systems, and the slow, methodical pace of long-term research.
And yet, this complexity shouldn’t breed paralysis or fear. If anything, it should empower us to think more clearly, to ask better questions, and to engage more fully with our own care. Because when we talk about risk — especially risks that are small, rare, or difficult to quantify — we’re really talking about context. About how materials behave not in isolation, but in people. Real, diverse, unpredictable people.
Titanium, in all fairness, deserves its place as the industry standard. It’s not perfect, but it has earned its reputation through decades of use, careful refinement, and overwhelmingly positive outcomes. It integrates with bone beautifully. It resists corrosion better than almost any other metal. And for millions, it simply works — invisibly, quietly, and indefinitely.
But as we’ve seen, the picture isn’t universally rosy. There are people for whom the implant never quite feels right. People who develop mysterious symptoms. People whose immune systems bristle at what’s supposed to be a biologically inert material. Are these the exception? Absolutely. But in medicine, exceptions matter — because they are often the first signals that our understanding still has edges left to explore.
This article hasn’t aimed to sway you toward or away from titanium. It’s aimed to make you a sharper participant in the conversation — to give you the tools to question, to notice, to weigh, and to decide.
Because that’s the future of medicine: not paternalistic, not one-size-fits-all, but partnership-based. It’s about looking at not just the material, but the person. Not just the implant, but the biology it enters, the immune system it meets, the environment it lives in over time.
It’s also about staying open to evolution. The science we trust today is always subject to revision — not because it was wrong, but because it was incomplete. And titanium, for all its benefits, will eventually give way to newer materials, smarter surfaces, and more personalized solutions. That’s not a rejection of what’s worked — it’s an invitation to build something even better.
So if you’re weighing the decision to get a titanium implant, or wondering whether the one you already have is affecting your health, here’s the most honest takeaway: trust the data, but trust your body, too. Seek out practitioners who respect both. Ask for clarity, and expect nuance in return. Medicine is at its best when it acknowledges uncertainty, not when it papers over it.
There’s a big difference between fear and informed skepticism. The former leads to avoidance. The latter leads to better questions, deeper understanding, and ultimately — better outcomes.
Stay curious. Stay critical. And most importantly, stay connected to your own lived experience. Because no one knows your body like you do.
And that — more than any scan, lab test, or abstract — is where real insight begins.