Can Microcurrent Cause Cancer? A Comprehensive Analysis
- Understanding Microcurrent Therapy
- Mechanisms of Action: How Microcurrent Affects Cells
- Evaluating the Evidence: Microcurrent Therapy and Cancer Risk
- Clinical Guidelines and Contraindications
- Potential Cellular Risks: Can Stimulation Promote Malignant Growth?
- Differences Between Microcurrent and Other Electrotherapies
- Use in Aesthetic Medicine: Safe or Overused?
- Animal Studies and Comparative Oncology
- Lack of Regulatory Oversight and Evidence Gaps
- Patient Populations at Risk: Survivors, Seniors, and Genetically Predisposed Individuals
- The Role of Practitioner Training and Clinical Judgment
- Consumer Education and Informed Consent
- Emerging Research and Experimental Models
- Integrating Microcurrent into Oncology-Safe Practice
- Legal and Ethical Considerations
- Weighing the Risks and Benefits
- Survivorship and Ongoing Monitoring for High-Risk Users
- FAQ
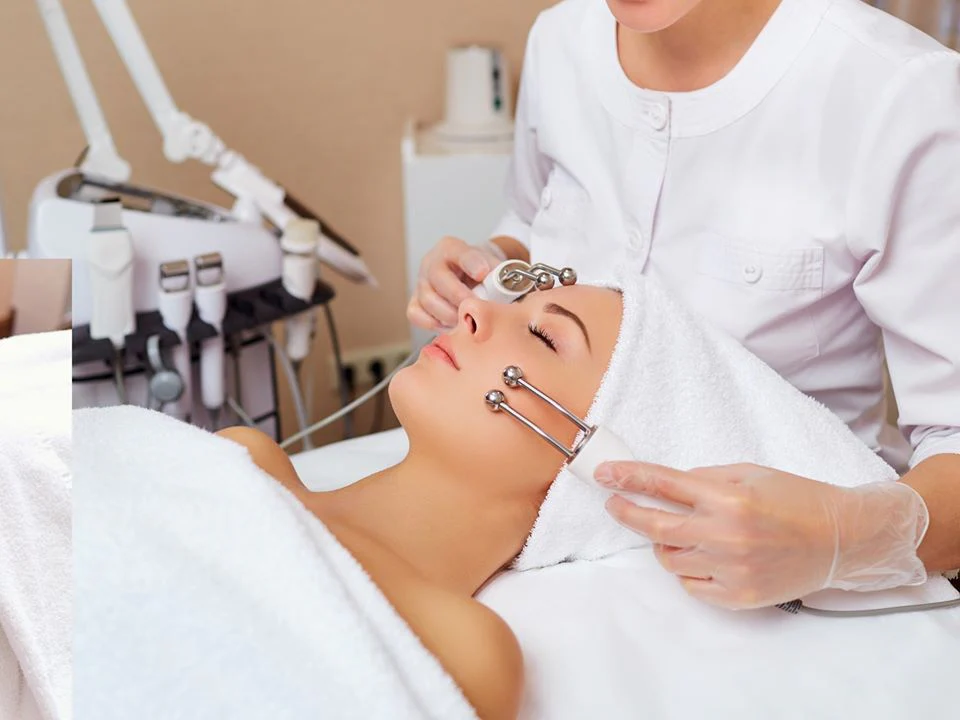
Understanding Microcurrent Therapy
Microcurrent therapy involves the application of low-level electrical currents to the body, typically in the microampere range. These currents are designed to mimic the body’s natural electrical signals, aiming to stimulate cellular activity and promote healing. Commonly used in physical therapy and cosmetic treatments, microcurrent therapy has been employed to address various conditions, including muscle pain, inflammation, and skin rejuvenation.
The therapy operates on the principle that electrical stimulation can enhance the production of adenosine triphosphate (ATP), the energy currency of cells. Increased ATP levels are believed to accelerate tissue repair and improve cellular function. Devices used for microcurrent therapy are often FDA-approved and are considered non-invasive and painless when administered correctly.
Mechanisms of Action: How Microcurrent Affects Cells
Microcurrent therapy’s primary mechanism involves the stimulation of cellular processes through low-level electrical currents. By enhancing ATP production, microcurrent therapy may facilitate various physiological responses, such as:
- Cellular Repair: Increased ATP can support the repair of damaged tissues by providing energy for cellular processes.
- Protein Synthesis: Enhanced energy availability may promote the synthesis of proteins necessary for tissue regeneration.
- Ion Exchange: Electrical stimulation can influence ion channels, affecting cellular communication and function.
These effects contribute to the therapeutic benefits observed in clinical settings, including reduced inflammation, improved circulation, and pain relief.
Evaluating the Evidence: Microcurrent Therapy and Cancer Risk
Current scientific literature does not establish a direct link between microcurrent therapy and the initiation or progression of cancer in healthy individuals. Studies have demonstrated the safety of microcurrent treatments when used appropriately, with minimal side effects reported. However, caution is advised for individuals with active cancer.
The concern arises from the therapy’s potential to stimulate cellular activity, which, in the context of cancer, could theoretically promote tumor growth. As a precaution, many practitioners recommend avoiding microcurrent therapy in areas with known malignancies or in patients undergoing cancer treatment. Further research is necessary to fully understand the implications and establish comprehensive guidelines.
Clinical Guidelines and Contraindications
While microcurrent therapy is generally safe, certain populations should exercise caution or avoid treatment altogether. Contraindications include:
- Active Cancer: Due to the potential risk of stimulating malignant cells, individuals with active cancer should avoid microcurrent therapy.
- Pregnancy: The effects of microcurrent therapy during pregnancy are not well-studied; thus, it is typically contraindicated.
- Pacemakers or Electronic Implants: Electrical currents may interfere with these devices, posing health risks.
- Epilepsy: Electrical stimulation could potentially trigger seizures in susceptible individuals.
- Recent Surgical Procedures: Healing tissues may be sensitive to electrical stimulation; consultation with a healthcare provider is recommended.
Patients should consult with qualified healthcare professionals before initiating microcurrent therapy to ensure it is appropriate for their specific health conditions.

Potential Cellular Risks: Can Stimulation Promote Malignant Growth?
Although microcurrent therapy is widely considered safe for general use, its influence on cellular activity has prompted questions about whether it could stimulate abnormal or malignant cell growth. The mechanism that enhances healing—through increased ATP production and cellular regeneration—might, in theory, support not only healthy cells but also precancerous or undiagnosed malignant ones.
This is particularly relevant when discussing conditions involving abnormal cell division, such as cancer. While no human studies conclusively demonstrate that microcurrent can trigger cancer, preclinical studies suggest that any electrical or metabolic stimulation in cancerous environments should be approached with caution. Importantly, in veterinary contexts like hyperthermia treatment for canine breast cancer, similar caution is practiced when considering cellular stimulation near tumors.
Therefore, individuals with a history of cancer or high genetic risk factors should undergo a thorough medical review before starting such therapies, even for cosmetic purposes.
Differences Between Microcurrent and Other Electrotherapies
It’s essential to distinguish microcurrent therapy from other forms of electrotherapy such as TENS (transcutaneous electrical nerve stimulation), EMS (electrical muscle stimulation), and galvanic treatments. While all use electric currents, the amplitude, waveform, frequency, and therapeutic intentions differ significantly.
Microcurrent delivers extremely low current—typically below the sensory threshold—while TENS and EMS involve higher, often muscle-contracting intensities. TENS is mainly for pain relief, EMS for muscle strengthening, and galvanic therapy for iontophoresis. These therapies have broader stimulation zones and different cellular impacts compared to the more targeted, subtle nature of microcurrent.
This distinction is critical when assessing potential oncologic risks. For example, TENS has been studied in cancer patients for palliative pain relief, while microcurrent’s role in oncology remains largely uninvestigated due to the potential risks of stimulating neoplastic tissue.

Use in Aesthetic Medicine: Safe or Overused?
Microcurrent therapy has surged in popularity within the aesthetic industry, especially for facial rejuvenation and skin tightening. Promoted as a non-invasive “facial workout,” it is used to stimulate facial muscles, improve skin tone, and reduce the appearance of wrinkles. Most at-home and professional devices operate in the 20–1000 microampere range and claim to improve lymphatic flow and collagen production.
However, the long-term impact of repeated electrical stimulation—particularly near lymph nodes, thyroid tissue, or other hormone-sensitive regions—has not been extensively studied. Dermatological associations generally endorse the short-term cosmetic benefits but advise caution in clients with thyroid conditions or suspicious skin lesions.
There is currently no evidence linking facial microcurrent therapy to skin cancer, but dermatologists emphasize the need for pre-treatment screening and moderation, especially in clients over 50 or with a personal history of cancer.
Animal Studies and Comparative Oncology
Some insights about the biological effects of electrical therapies can be drawn from veterinary medicine. In animal models, microcurrent and electrotherapies have been used to accelerate wound healing, reduce inflammation, and improve post-surgical recovery. However, studies involving tumor-bearing animals have raised questions about how such therapies may interact with neoplastic processes.
For instance, in the study of spinal cancer in dogs, researchers explicitly avoid localized electrotherapy near the spine when tumors are present. The reasoning is that even small energetic changes could influence tumor metabolism or growth kinetics.
While animal models cannot fully predict human responses, they do offer preliminary safety frameworks. Until more definitive human data are available, lessons from comparative oncology suggest that microcurrent therapy should be avoided over known or suspected malignancies—even in asymptomatic individuals.
Lack of Regulatory Oversight and Evidence Gaps
Despite its growing popularity, microcurrent therapy exists in a regulatory gray zone, particularly in aesthetic and at-home consumer markets. While some devices are FDA-cleared for general cosmetic use or physical therapy purposes, this clearance is not equivalent to formal approval of safety in oncology or high-risk medical populations.
Most microcurrent devices are classified under Class I or II medical devices, meaning they are considered low-risk when used as directed. However, few long-term clinical trials have evaluated their effects on DNA integrity, mitochondrial stress, or neoplastic cell behavior. As a result, public health authorities do not currently issue comprehensive guidance on microcurrent use in people with prior cancer history.
This regulatory gap mirrors concerns raised in pharmacological oncology, where powerful therapies like the TCHP regimen for HER2-positive breast cancer are rigorously evaluated and monitored for systemic effects. Patients who undergo TCHP chemotherapy are often advised to avoid external stimulatory interventions during and after treatment due to immune system sensitivity and potential cellular reactivation. Such caution could reasonably be extended to microcurrent use, especially in oncology survivors.
Patient Populations at Risk: Survivors, Seniors, and Genetically Predisposed Individuals
While microcurrent therapy is marketed to a broad audience, certain subgroups may require additional caution due to underlying vulnerabilities. Cancer survivors, particularly those who have undergone radiation or chemotherapy, may experience altered tissue integrity, immunological shifts, or subclinical cellular changes that heighten their sensitivity to further stimulation.
Seniors with age-related cellular degradation or impaired DNA repair mechanisms might also be at higher theoretical risk. Similarly, individuals with BRCA1/2 mutations or other known genetic predispositions for cancer are advised to limit therapies that could enhance cellular proliferation, even minimally.
Although the majority of consumers may use microcurrent without harm, these at-risk populations would benefit from prior clearance by a physician. A personalized, cautious approach ensures that safety is not compromised in pursuit of cosmetic or physical therapy outcomes.
The Role of Practitioner Training and Clinical Judgment
The risk profile of microcurrent therapy is heavily influenced by the skill and awareness of the practitioner delivering the treatment. Licensed professionals who understand the nuances of physiology, contraindications, and patient history are better equipped to screen for potential red flags before proceeding with therapy.
Unfortunately, many spa or at-home devices are operated by individuals without formal medical education. In these cases, preexisting cancers or conditions may go undetected, increasing the chance of inadvertent stimulation of pathological tissue.
Training programs for microcurrent technicians often focus on device operation and aesthetic outcomes rather than cellular biology or oncology screening. Raising standards for certification and encouraging clinical collaboration—especially with dermatologists, oncologists, and physical therapists—would enhance the safety profile of microcurrent therapies across the board.
Consumer Education and Informed Consent
One of the most effective ways to ensure safe microcurrent therapy is through informed, science-based patient education. Users should be aware that although microcurrent appears gentle and non-invasive, it is not entirely biologically neutral. It interacts with cells in subtle yet significant ways that are still not fully understood, particularly over long-term or repeated exposures.
Before initiating therapy, especially in high-risk zones like the neck, chest, or jawline, clients should be informed of potential contraindications and asked about personal or family cancer history. In clinical settings, informed consent forms should include mention of the lack of data regarding cancer risk, particularly for individuals in remission or with genetic predispositions.
Medical aesthetic practices that prioritize transparency build stronger patient trust and encourage safer, evidence-aligned care. Until large-scale longitudinal studies are available, prudence remains a responsible guiding principle.
Emerging Research and Experimental Models
Although microcurrent therapy has been widely adopted in physical therapy and aesthetics, there is a noticeable lack of rigorous oncological research examining its long-term effects. This gap is slowly being addressed through in vitro studies and early-stage animal models that explore how low-level electrical currents interact with biological tissues — both healthy and malignant.
Some studies using human fibroblasts and keratinocytes in laboratory settings have shown that microcurrent can enhance cellular proliferation, ATP synthesis, and protein expression. While beneficial for healing, these same mechanisms may raise theoretical concerns about overstimulation in abnormal cells. A few experimental models using neoplastic cell lines have demonstrated that specific current intensities can influence gene expression and possibly promote angiogenesis, though these findings are context-dependent and not fully reproducible across models.
Animal studies offer additional insight. In veterinary medicine, electrotherapies are cautiously excluded in cases of active tumors — for example, spinal cancer in dogs is typically treated with radiation or surgery, not localized stimulation, due to concerns about metastatic activation. These findings do not prove causality but highlight the need for better-designed human trials to clarify safety parameters and risk thresholds in high-risk populations.
Integrating Microcurrent into Oncology-Safe Practice
Safe clinical integration of microcurrent therapy, particularly in settings that serve cancer survivors or individuals with complex histories, requires structured protocols and interdisciplinary collaboration. Oncology-safe microcurrent use is possible but must be customized to minimize risk, avoid high-risk anatomical zones, and prioritize transparency.
An oncology-safe protocol includes thorough pre-treatment screening: full medical history intake, clarification of past cancer treatments, and identification of vulnerable tissue zones. For example, patients with a history of breast cancer should avoid microcurrent near axillary lymph nodes or chest tissue. Survivors of head and neck cancers should not receive facial stimulation without oncologist clearance.
In clinical settings, therapists increasingly adopt integrative approaches, combining microcurrent with passive modalities like LED therapy or gentle lymphatic massage in lower-risk zones. Scheduling is also key — patients undergoing active chemotherapy, immunotherapy, or radiation should defer microcurrent until all systemic treatment and immune stabilization have concluded. When used responsibly, microcurrent may support recovery from treatment-related fatigue or muscle tension — but only with oncology supervision.
Legal and Ethical Considerations
The legal and ethical landscape surrounding microcurrent therapy is becoming more complex as the therapy expands beyond medical offices into medspas, beauty clinics, and consumer households. Most countries classify microcurrent devices as Class I or II medical devices, meaning they pose minimal risk when used appropriately — but that classification does not account for vulnerable populations such as cancer survivors or individuals with implants.
Legally, misrepresenting a device’s safety — particularly to high-risk clients — could result in liability. In the absence of strong federal regulation, it falls to clinics and manufacturers to provide accurate risk disclosures. Ethically, providers have a responsibility to uphold informed consent standards: patients must be told what is known, what is unknown, and what cannot be guaranteed. This is especially true in wellness settings where medical supervision is minimal or absent.
Marketing claims that oversell benefits or understate contraindications violate principles of beneficence and non-maleficence. Aesthetic professionals and physiotherapists should be trained in ethical risk communication, proper documentation, and referral protocols. The presence of comorbidities such as cancer history, autoimmune disease, or genetic predisposition should automatically prompt referral to a physician before treatment begins.
Weighing the Risks and Benefits
Microcurrent therapy holds promise as a non-invasive tool for tissue repair, pain relief, and aesthetic rejuvenation. For many healthy individuals, the benefits — improved circulation, ATP production, muscle tone — are real and well-supported by small clinical trials and user experience. However, the mechanisms that make microcurrent effective are also the ones that raise concerns in oncologic contexts.
There is currently no clinical evidence that microcurrent causes cancer. However, due to the biological possibility that it may accelerate cellular growth in compromised or high-risk tissues, it should not be used indiscriminately. Until we have robust long-term data, the safest path is conditional use: with clear contraindications, risk stratification, and medical oversight.
For high-risk individuals — including cancer survivors, those with strong family histories, or patients undergoing immunotherapy — microcurrent may not be the appropriate therapy. For others, especially those seeking post-injury support or facial toning under supervision, it may be a valuable adjunct. As always, informed choice backed by transparent science must guide the decision.
Survivorship and Ongoing Monitoring for High-Risk Users
For individuals who have had cancer, particularly those in remission from hormone-sensitive, HER2-positive, or aggressive subtypes, long-term vigilance is a key element of survivorship care. Microcurrent therapy is not inherently off-limits for these individuals, but it must be approached with heightened caution.
Survivors should maintain regular follow-ups with their oncology teams and inform them before pursuing aesthetic or rehabilitative treatments involving electrostimulation. For instance, patients treated with TCHP chemotherapy for HER2-positive breast cancer are advised to avoid energetic or stimulatory therapies on or near the torso until cardiac and cellular stability are well-documented.
If microcurrent is eventually approved for use by the survivor’s care team, it should begin at the lowest effective intensity, in the safest anatomical areas, and under trained professional supervision. Periodic reassessment should be scheduled to monitor skin integrity, lymph node response, and systemic tolerance. Survivor safety is never static — it evolves with biology, history, and time.
FAQ
Can microcurrent therapy cause cancer in healthy individuals?
No current scientific evidence suggests that microcurrent therapy causes cancer in otherwise healthy individuals. The therapy uses extremely low electrical currents and is considered safe when used correctly. However, because it stimulates cellular activity, some researchers and clinicians recommend caution in people with known risk factors or a history of malignancy.
Is it safe to use microcurrent devices if I had cancer in the past?
This depends on several factors, including the type of cancer, how long you’ve been in remission, and the area being treated. Many oncologists recommend avoiding microcurrent over or near previous tumor sites. Consultation with your oncology team is strongly advised before resuming or starting this therapy post-cancer.
Can microcurrent make existing cancer grow faster?
While there is no conclusive proof that microcurrent accelerates tumor growth, the theoretical risk exists because the therapy increases ATP and may promote cellular regeneration. In people with active or undiagnosed cancer, this stimulation could potentially support tumor progression, which is why it’s generally contraindicated in oncology patients.
Why is microcurrent therapy not recommended during cancer treatment?
During cancer treatment, the body is undergoing systemic changes, and the immune system is often compromised. Microcurrent’s stimulatory effects could interfere with these processes or unintentionally energize cancerous cells. As a result, most practitioners avoid applying electrical stimulation during chemotherapy, radiation, or immunotherapy.
Is it safe to use microcurrent on the face or neck if I’ve had thyroid cancer?
Caution is advised when treating areas near hormonally sensitive tissues such as the thyroid, especially in patients with a history of thyroid cancer. Although microcurrent devices used in cosmetic procedures are low-intensity, it’s better to avoid these zones unless your endocrinologist or oncologist approves.
How is microcurrent different from TENS or EMS?
Microcurrent therapy operates at significantly lower currents than TENS (used for pain) or EMS (used for muscle contraction). TENS and EMS are designed to stimulate nerves or muscles more aggressively, while microcurrent aims to mimic natural cellular signaling. Each modality has different applications and biological effects.
Can microcurrent be used during physical therapy for injury recovery?
Yes, in individuals without contraindications, microcurrent therapy is frequently used in physical therapy to aid in muscle healing, reduce inflammation, and accelerate tissue repair. However, thorough medical screening should still be conducted to rule out any underlying risks, especially in older patients or those with a cancer history.
What are the long-term risks of using microcurrent for cosmetic purposes?
Long-term safety data is limited. While no adverse effects have been definitively linked to prolonged microcurrent use, the absence of large-scale longitudinal studies means unknown risks cannot be ruled out—particularly with repeated use over hormone-sensitive or previously irradiated areas.
Is there a link between microcurrent therapy and breast cancer?
There is no established link. However, women with a personal or family history of breast cancer are generally advised to avoid microcurrent therapy over the chest area. Medical aesthetic practices often exclude the breast zone from treatment zones as a precautionary measure.
How do I know if a device is safe to use at home?
Only use FDA-cleared or CE-marked devices, and always follow the manufacturer’s instructions. Look for transparency in technical specifications, including current range, and avoid using the device over areas with implants, moles, or suspicious lesions. When in doubt, consult a dermatologist or physician before use.
Can microcurrent be used after radiation therapy?
Radiated tissues can be more fragile and respond differently to electrical stimulation. While some rehabilitative protocols cautiously use electrotherapy post-radiation, microcurrent should be avoided over radiated areas unless cleared by a radiation oncologist or specialist.
Are there warning signs that microcurrent therapy is not suitable for me?
Yes. If you have a pacemaker, are pregnant, have epilepsy, or have had recent surgery or cancer, you should avoid microcurrent unless your doctor advises otherwise. Skin changes like swelling, redness, or discomfort after treatment should also prompt medical evaluation.
How should survivors of HER2-positive cancer approach microcurrent use?
Survivors of HER2-positive cancers treated with intensive regimens like TCHP should avoid microcurrent therapy during and immediately after treatment. Once cardiac function and tissue integrity have stabilized, a physician may approve limited use, but conservative protocols are advised.
Are animal studies relevant to understanding microcurrent risks?
Yes. Animal studies, particularly in canine oncology, have shown that electrical stimulation near tumors can pose risks. In cases like spinal cancer in dogs, electrotherapies are deliberately avoided. These insights support the cautionary approach adopted in human medicine.
What should I ask my provider before starting microcurrent therapy?
Ask about their training, how they screen for contraindications, what protocols they follow for high-risk clients, and whether they coordinate with medical professionals. Don’t hesitate to share your medical history openly, especially regarding cancer, implants, or hormone-sensitive conditions.









