Breast Cancer Index Test: Should You Extend Endocrine Therapy?
- Part 1: Introduction to the Breast Cancer Index (BCI) Test
- Part 2: Scientific Foundations of the Breast Cancer Index (BCI) Test
- Part 3: Clinical Applications and Decision-Making with the Breast Cancer Index Test
- Part 4: Eligibility Criteria for the BCI Test
- Part 5: Procedure and Interpretation of Results
- Part 6: How BCI Compares to Other Genomic Tests
- Part 7: Clinical Evidence and Validation Studies
- Part 8: Cost, Insurance, and Accessibility
- Part 9: Patient Perspectives and Shared Decision-Making
- Part 11: Frequently Asked Questions (FAQs)
- Closing Thoughts
Introduction to the Breast Cancer Index (BCI) Test
If you or someone close to you has been diagnosed with hormone receptor-positive (HR+), early-stage breast cancer, chances are you’ve heard a flurry of terms—Oncotype DX, MammaPrint, Ki-67, and maybe, if your oncologist is especially up-to-date, something called the Breast Cancer Index test. The BCI isn’t just another acronym in the cancer lexicon. It’s part of a revolution: one where we treat individuals, not categories; where treatment decisions are data-driven, not guesswork; and where “what if?” gets replaced with “what now?”
So what exactly is the Breast Cancer Index test—and why is it suddenly showing up in major treatment conversations?
At its core, the BCI test is a genomic assay. That means it doesn’t look at your tumor the way a microscope might, assessing its size or shape or how fast it seems to be growing. Instead, it looks at the genes inside the tumor cells—the messages those cells are sending, the blueprints they’re following. Think of it as listening in on a conversation your cancer cells are having behind closed doors. What are they saying about their long-term plans? Are they planning to come back five years from now? Are they likely to respond if we continue endocrine therapy for another five?
You might be thinking, Didn’t we already have tests like this? Yes—and no. Tests like Oncotype DX and MammaPrint have indeed transformed how we approach chemotherapy decisions, especially right after diagnosis. But they don’t quite tackle the question the BCI was built for: whether it’s worth extending endocrine therapy beyond the standard five years. That’s the BCI’s sweet spot.
Let’s put this in context. A woman finishes five years of tamoxifen or an aromatase inhibitor. She’s tired, maybe dealing with side effects like joint pain, hot flashes, or fatigue. Her cancer hasn’t returned. Life, finally, feels steady. And then comes the question: Should she stay on this therapy for five more years?
This is where the BCI test earns its keep.
By analyzing a set of 11 specific genes from the original tumor tissue—no new biopsy required—it provides two key pieces of information:
- How likely is this person to experience a late distant recurrence (in years 5 to 10)?
- Is there a real benefit to continuing endocrine therapy beyond the five-year mark?
These are not trivial questions. Endocrine therapy can have a substantial impact on quality of life. It may prevent recurrence—but for some, it might also add years of unnecessary discomfort. Until recently, decisions about extended therapy were made with broad strokes, based on clinical features like tumor size or lymph node involvement. The BCI adds a finer brush, letting clinicians and patients paint a more detailed, individualized picture.
Now, you may wonder, If this test is so useful, why isn’t everyone using it? Great question—and one with a nuanced answer. BCI is still making its way into routine practice. Not all oncologists are yet comfortable navigating its implications. Some are more familiar with earlier-generation genomic tools. Others might be waiting for broader consensus in guidelines or more long-term outcomes data. But the momentum is building—and with good reason.
What sets BCI apart is its predictive ability. Most genomic assays are either prognostic (they tell you the likelihood of recurrence) or predictive (they tell you how likely a treatment is to work). The Breast Cancer Index is both. It can tell you not just what might happen, but what might help.
So who’s it for? Predominantly postmenopausal women with HR+ breast cancer, who’ve completed five years of endocrine therapy and are weighing their next move. It’s not designed for newly diagnosed patients deciding on initial treatment, nor for those with HER2-positive or triple-negative disease. It occupies a specific, important niche.
This test doesn’t make the decision for you—but it makes the decision-making smarter. It brings clarity to a gray area, and in oncology, that’s no small thing.
This test is one of several tools used to individualize long-term treatment plans, and it’s often mentioned alongside metrics like Ki-67 proliferation index.
In the sections that follow, we’ll break down how the test works, what the science says, how to interpret the results, and how it stacks up against other tests you might have encountered. But for now, hold onto this: the BCI test exists because medicine is shifting away from one-size-fits-all and toward what’s right for you. If that sounds like progress, that’s because it is.
Scientific Foundations of the Breast Cancer Index (BCI) Test
Let’s dig into the engine room. You’re probably wondering: how does the Breast Cancer Index actually work? How does a small slice of tumor tissue—likely stored in a lab somewhere since your surgery—suddenly hold the answers to whether you should extend hormone therapy?
It all starts with gene expression profiling. If that phrase conjures images of white-coated scientists staring into microscopes, think bigger. This isn’t microscopy; it’s molecular eavesdropping. The BCI doesn’t just look at the tumor’s appearance, it listens to its behavior. It’s analyzing which genes are actively being transcribed into RNA, and from that, it infers what the cancer cells are trying to do next. Are they gearing up for a comeback? Or settling into dormancy?
The BCI test evaluates the expression of 11 genes taken from your original tumor sample—genes that were always there but only now being asked the right questions. These genes are grouped into two key components: the Molecular Grade Index (MGI) and the HOXB13/IL17BR (H/I) ratio. Let’s unpack both without veering into graduate-level biochemistry.
The MGI is all about aggressiveness. Think of it as the tumor’s “momentum meter.” It measures how fast the tumor cells were dividing, how much energy they were burning to grow and spread. The five genes in this group give insight into the tumor’s natural proliferation speed. If the score is high, it suggests a more ambitious cancer—one that might warrant extra years of suppression through hormone therapy.
The H/I ratio, on the other hand, is the test’s crystal ball. This duo of genes (HOXB13 and IL17BR) is less about the tumor’s brute force and more about its subtle signals—specifically, how sensitive it is to estrogen and, by extension, to anti-estrogen therapy. If this ratio comes out high, it tells us the tumor may still be influenced by hormonal pathways and that extending endocrine therapy might actually work. A low ratio? Maybe not worth the toll.
The magic of BCI lies in the combination of these two scores. It’s not just asking, “Is this cancer likely to come back?” It’s also asking, “If it does try, will more therapy help stop it?” That distinction matters—immensely. Because no patient wants to undergo years of medication and its accompanying side effects (think hot flashes, bone thinning, joint stiffness, mood swings) if the return on investment is negligible.
Some tests stop at prognosis. They tell you what might happen, but they can’t tell you what to do about it. The BCI test goes one step further: it connects prognosis with action. That predictive layer—identifying who’s actually likely to benefitfrom more treatment—is why the BCI is carving out a space of its own.
Now, here’s a question many patients and even some clinicians ask: why only 11 genes? It seems modest compared to the 21 or 70 genes analyzed in other tests. But in this case, less is more. The selected genes in BCI were not chosen at random; they were identified through rigorous research, carefully validated, and shown to produce meaningful, reproducible insights specifically about long-term recurrence and therapy response. In other words, these 11 aren’t just any 11—they’re the ones that matter most for this decision point.
It’s also worth noting that BCI uses formalin-fixed, paraffin-embedded tissue—the standard way tumors are preserved after surgery. That means if you’re several years out from diagnosis, you don’t need a new procedure or biopsy. The path lab dusts off your sample, sends it to the BCI processing lab, and the analysis begins from what’s already available. That’s convenience meets precision.
If you’re thinking this all sounds incredibly high-tech for something happening behind the scenes of your care—you’re right. But that’s precisely the point. Genomic assays like the BCI are part of a new generation of tools that make invisible information visible. They’re not replacing your doctor; they’re sharpening their decision-making ability. When faced with the deeply personal question of whether to keep taking medication for another five years, “gut instinct” shouldn’t be the only guide. Now, it doesn’t have to be.
In the next section, we’ll explore what this test means in the clinic—how it translates from gene expression into real-world decisions, and how it’s reshaping the conversations between oncologists and their patients. Because at the end of the day, science is only as useful as its application—and that’s where things get personal.
Part 3: Clinical Applications and Decision-Making with the Breast Cancer Index Test
By now, we’ve established that the Breast Cancer Index (BCI) is a genomic test built to answer two deeply specific questions: What is the chance of my cancer coming back between years 5 and 10? and Would taking more endocrine therapy actually reduce that risk? But how exactly do these answers impact real-world clinical decisions? What does this test actually do in the day-to-day life of a breast cancer patient and her care team?
Let’s walk through the fork in the road that most BCI candidates face. Imagine this: You’re a postmenopausal woman with early-stage, estrogen receptor-positive (ER+), HER2-negative breast cancer. You’ve dutifully completed five years of endocrine therapy—tamoxifen, letrozole, anastrozole, or another aromatase inhibitor. You’ve gone through the bone aches, the sleep disruptions, the mood swings, and the never-ending hot flashes. Now the finish line is in sight.
But your oncologist sits you down and says: “We need to talk about another five years.”
This isn’t hypothetical. For many women in this position, there’s a medically plausible case to be made for extended endocrine therapy. Statistically speaking, ER+ breast cancers have a slower, more insidious pattern of recurrence. A significant portion of recurrences actually happen after year five. That’s why clinicians often consider a longer duration of treatment.
The trouble is, until recently, that decision was made based on guesswork. Guidelines would weigh your tumor’s size, whether lymph nodes were involved, how old you are, and a handful of other characteristics. But none of that is particularly precise when it comes to predicting late recurrence, and none of it could tell you whether the second leg of endocrine therapy would actually help you—not just women like you, but you.
This is the clinical gap the BCI test fills.
When a BCI test is ordered, it delivers two essential pieces of information. First, it gives a risk score—your personalized percentage likelihood of a distant recurrence between years 5 and 10. Second, and this is the real clincher, it provides a yes/no indicator for whether extended endocrine therapy is likely to reduce that risk in a meaningful way.
It’s not just a snapshot of risk; it’s a recommendation tethered to action.
Let’s say your BCI comes back showing a low risk of late recurrence and no significant benefit from more therapy. That’s a green light to stop endocrine treatment at year five, reclaim your body from the side effects, and move forward with confidence. But if your test shows a higher risk—and also signals that extended therapy could actually move the needle on that risk—then continuing becomes not just an option but a rational, personalized choice.
This kind of targeted clarity is a game changer, especially in a decision space that used to be muddied by generalizations.
You might be asking, What if my doctor already thinks I’m high risk based on traditional factors? Shouldn’t that be enough? Here’s the interesting twist: clinical-pathologic features like tumor grade, lymph node involvement, and size don’t always align with genomic behavior. A tumor that looks aggressive under the microscope might actually have a quiet genomic profile. And vice versa. The BCI offers insight from a different angle—biology over appearance.
And what about scenarios where the test contradicts what you expected? That happens more often than you’d think. A woman with several positive nodes may still test low on BCI. Another with a small, node-negative tumor might test high. It’s not always intuitive, and that’s precisely why the test matters. It catches what eyes—and even decades of clinical experience—can miss.
It’s also important to understand that the BCI doesn’t exist in a vacuum. It’s a tool, not a verdict. Oncologists use it alongside other factors: your overall health, your tolerance to medication, your personal feelings about ongoing treatment. For some, even a modest reduction in recurrence risk is worth five more years of side effects. For others, it isn’t. The test helps anchor that conversation in data rather than uncertainty.
Another thing worth clarifying: the BCI isn’t about chemotherapy decisions. If you’re still in the early stages of treatment planning and wondering whether to do chemo, that’s a question for other assays like Oncotype DX or MammaPrint. The BCI comes into play later—once chemo is long done, once endocrine therapy has run its initial course, and the question becomes: Do I keep going, or do I stop?
This is the kind of moment where knowledge becomes empowerment. The BCI doesn’t just offer results; it offers reassurance. Reassurance that your decision—whether it’s to continue therapy or not—is grounded in evidence tailored to your biology, not just the law of averages.
Not everyone opts to extend therapy, of course, and our guide on Refusing Hormone Therapy breaks down reasons, risks, and lived experiences.
In the next section, we’ll take a closer look at who this test is really for. Not everyone qualifies for BCI testing, and it’s important to understand its limitations as well as its strengths. But if you’re nearing the five-year mark and facing that gnawing question—what now?—then you’re already standing at the crossroads where the BCI may hold the most power.
Part 4: Eligibility Criteria for the BCI Test
So now that we’ve established what the Breast Cancer Index test does, the next logical question is: Who gets it? Is it available to everyone with breast cancer? Could it apply to someone who’s just been diagnosed? Or is it only useful for a certain slice of patients at a particular moment in their journey?
Let’s clear the air: the BCI test isn’t for everyone. And that’s not a flaw—it’s intentional. This is a test with a very specific clinical purpose, designed to answer a very particular question. As we’ve already seen, the BCI exists to guide decisions about extended endocrine therapy—meaning, therapy that goes beyond the standard five years already recommended after initial treatment. So by definition, it’s only relevant when you’ve already reached (or are approaching) that five-year milestone.
Here’s the most common scenario: a postmenopausal woman with early-stage, hormone receptor-positive (HR+), HER2-negative breast cancer who has completed five years of endocrine therapy. That’s the sweet spot—the clinical profile for which the BCI has been validated, studied, and approved. It’s not meant to guide chemotherapy decisions (other genomic tests like Oncotype DX and EndoPredict handle that), and it’s not typically used at the point of diagnosis. Instead, it steps into the spotlight when you’re five years into your journey and wondering: Should I keep taking this medication for five more?
What about men with breast cancer? What about premenopausal women? What if you had a recurrence already? What if your tumor was HER2-positive?
These are excellent questions—and they get to the heart of what precision medicine is (and isn’t). BCI is currently validated only for postmenopausal women. That means the evidence for using it in other groups—men, younger women, or patients with different tumor biology—simply isn’t strong enough yet to support its use as standard care. That doesn’t mean it won’t be in the future. But right now, the test’s reliability and interpretability are grounded in data from postmenopausal populations.
And yes, BCI also requires that your cancer be HER2-negative. That’s because HER2-positive tumors behave very differently from HR+ ones—they’re often more aggressive, respond to different therapies, and follow distinct recurrence patterns. Mixing HER2 biology with BCI’s gene expression models would confound the results. The test just isn’t built to address the HER2 pathway.
Then there’s the question of tumor stage and nodal involvement. You might have heard that the test is mainly for women with node-negative cancers. That’s partly true—but the picture’s evolving. Originally, BCI was developed for node-negative patients, but several studies have since demonstrated its usefulness in women with 1–3 positive lymph nodes—provided they still meet the other criteria. So if you had a small number of positive nodes but are otherwise a candidate (ER+, HER2−, postmenopausal, five years into therapy), your oncologist may still consider ordering the test.
Some patients also ask: What if I’m in year 4, not quite at year 5 yet—is the test still relevant? The answer depends on clinical judgment. The test can be ordered slightly before the five-year mark, especially if the results will meaningfully inform whether you transition into extended therapy once you cross that threshold. In other words, the decision doesn’t have to happen at the exact anniversary. It just needs to be timed close enough to matter.
And perhaps the most pragmatic question of all: Is it too late if I’m already in year 6 or 7 of therapy? Not necessarily. Some patients who’ve continued therapy by default, without strong guidance, may still benefit from a retrospective evaluation. If side effects have become intolerable or you’re questioning whether to go the full 10 years, a BCI test could still help recalibrate that plan. Again, it’s about where you are in your treatment arc and whether you’re still at a decision point.
One thing to keep in mind: while your oncologist will ultimately determine if you’re a candidate, you don’t need to be passive in this process. If you fit the general criteria and you’re facing the extended therapy decision, it’s perfectly reasonable to ask, “Would the BCI test help clarify what’s right for me?” Informed patients often drive better outcomes—not just medically, but emotionally too. Knowing why you’re continuing (or discontinuing) therapy is a vastly different psychological experience from simply doing what the calendar dictates.
In the next section, we’ll look more closely at how the test is actually performed and how the results are interpreted. What does it feel like to go through the BCI process? What can you expect to learn—and how should you use that information? Spoiler: it’s not just about getting a number. It’s about shifting from uncertainty to strategy.
Procedure and Interpretation of Results
Let’s say your oncologist has recommended the Breast Cancer Index test. Or maybe you brought it up—because you’ve done your homework, you’re five years into endocrine therapy, and you’d like more than just a shrug when deciding whether to go another five. So what happens next? What exactly does the process look like? And when the results come in, how are they actually used?
Here’s the reassuring part: the BCI test is noninvasive. There’s no need for a new biopsy, no blood draw, no awkward procedure. The test is performed using the tissue that was already removed during your original surgery—usually preserved in what’s called a formalin-fixed, paraffin-embedded (FFPE) block. If you’ve never heard of that, you’re not alone—but it’s the standard way pathology labs store tumor tissue after surgery. In many cases, your hospital still has that tissue sitting in its archives, and the BCI lab simply requisitions a small section from it.
Once that sample is secured, it’s shipped off to a specialized genomic testing lab (often under the Hologic/biotheranostics umbrella). From there, technicians extract the RNA from the preserved tumor cells and analyze the expression levels of those 11 critical genes we talked about earlier—the ones that speak to the tumor’s biology and estrogen responsiveness.
Turnaround time? Usually around 10 to 14 days. Not exactly instant, but fast enough to fit neatly into most follow-up appointments or upcoming treatment decisions.
But let’s talk about what you’ll actually see when the results come back—because this is where the BCI shifts from abstract science to actionable insight.
You’ll typically receive a report that includes two key elements:
- A Risk Score for Late Distant Recurrence – This is expressed as a percentage. For example, your report might say you have a 4.2% risk of distant recurrence between years 5 and 10 if you stop endocrine therapy. This number is derived from the gene expression patterns in your tumor and reflects how likely it is that the cancer could return in a distant organ (like bone or lung) after five years.
- Extended Endocrine Therapy Benefit Indicator – This is a binary output: either “Likely to Benefit” or “Unlikely to Benefit” from continuing endocrine therapy beyond year five. This is where the test flexes its predictive muscle. It doesn’t just say whether you’re at risk—it tells you whether treatment can reduce that risk.
You might ask, What if I have a high recurrence risk but the test says I’m unlikely to benefit from more therapy? That’s a crucial scenario to consider. It means that although your risk isn’t negligible, the specific biology of your tumor suggests that additional hormone therapy won’t significantly lower it. That may sound counterintuitive, but it’s exactly the nuance that makes BCI valuable. In this case, pushing through another five years of therapy might only expose you to side effects—without offering much protection in return.
On the flip side, you could have a relatively modest risk—say, around 6%—but the test indicates a clear benefit from continued therapy. That small risk might be just enough to tip the scales in favor of extending, especially if the treatment is tolerable and your personal philosophy leans toward minimizing any recurrence risk, however small.
This interplay—between recurrence risk and therapy benefit—is the central conversation that the BCI test empowers. It’s not about making a blanket recommendation; it’s about aligning your clinical risk with your biological likelihood of benefiting from intervention.
The report is typically reviewed with your oncologist, who will walk you through the numbers, the implications, and the options. Don’t expect the test to dictate a single course of action. Medicine isn’t math—it’s a dialogue. BCI provides a refined data point to inform that dialogue, not to replace it.
Another common question: Are the results absolute? Should I make a life-altering decision based on this one test? The answer is: not in isolation. Like any clinical tool, BCI is meant to be integrated with your broader medical picture—age, menopausal status, previous side effects, bone health, comorbidities, personal risk tolerance, even your life plans. One woman’s “yes” might be another’s “no,” even with the exact same BCI results.
And what about the emotional side of interpreting these results? That’s worth pausing on. For some, a “low risk, no benefit” result is liberating—it’s a permission slip to stop treatment and move forward. For others, even a 5% risk might feel too high, especially after years of vigilance. That’s why BCI isn’t just a test; it’s a catalyst for a deeper, personalized conversation—one that ideally includes not just your doctor, but also your support system, your goals, and your comfort level with ambiguity.
In the next section, we’ll zoom out and see how BCI stacks up against other genomic tests on the market. Because while it’s tempting to think of these tools as interchangeable, they each have unique purposes—and understanding those distinctions is key to making empowered decisions. After all, choosing the right test is part of choosing the right path forward.
How BCI Compares to Other Genomic Tests
Now that we’ve peeled back the layers of what the Breast Cancer Index (BCI) does and how, it’s natural to wonder how it stacks up against other tests in the breast cancer toolbox. And that’s an important question—because let’s be honest, the moment you hear about one genomic assay, you quickly realize there are several others already in play: Oncotype DX, MammaPrint, EndoPredict, Prosigna. It’s a crowded field, and they all sound remarkably sophisticated. But here’s the catch: they are not interchangeable. Each test answers a different clinical question, and knowing the difference can save you from both confusion and potentially unnecessary testing.
So how does BCI distinguish itself?
Let’s start with Oncotype DX, arguably the most well-known and widely used test in early breast cancer. It analyzes the activity of 21 genes and provides a Recurrence Score that helps predict the likelihood of cancer returning within the first five years—and whether chemotherapy would help reduce that risk. It’s used right after diagnosis, typically before any adjuvant therapy is given, to decide if chemo should be added to endocrine therapy. In short: Oncotype DX is a prognostic and predictive tool for chemo decisions at initial diagnosis. That’s a very different point in the timeline than where BCI operates.
BCI, in contrast, enters the picture much later—when you’re at or near the end of the initial five-year endocrine therapy course. Oncotype might have helped decide whether you even needed chemo; now, BCI helps you decide whether you need five more years of hormone therapy. So even though both are genomic tests for HR+ breast cancer, they’re deployed at different stages with different goals.
Then there’s MammaPrint, a 70-gene signature assay developed in Europe. Like Oncotype DX, it’s used near diagnosis to assess recurrence risk and determine the potential benefit of chemotherapy. MammaPrint categorizes tumors as either “low risk” or “high risk” of recurrence over a 10-year span—but it doesn’t provide specific guidance about the usefulness of extended endocrine therapy. It gives a broader, population-level picture, but not the tailored post-treatment insight that BCI provides.
You might also hear about EndoPredict and Prosigna (PAM50), which sit somewhere between Oncotype and BCI in terms of timing. Both give longer-term recurrence risk estimates and are sometimes used to guide decisions about the duration of endocrine therapy. However, unlike BCI, they are primarily prognostic—they can tell you if your risk of recurrence is high or low, but they don’t directly indicate whether more hormone therapy will provide additional benefit. That subtle difference is critical.
Why does it matter whether a test is prognostic or predictive? Because it’s one thing to be told your cancer might come back. It’s quite another to know whether additional treatment will change that outcome. BCI is currently the only testwith data robust enough to be considered predictive for extended endocrine therapy in postmenopausal women. That’s not marketing spin—it’s an important clinical distinction.
Here’s an analogy: imagine you’re at risk for a car accident on a winding road. A prognostic test says, “Yes, there’s a risk.” A predictive test says, “If you install this high-tech brake system, your risk will go down by X percent.” BCI is the brake-system insight. It doesn’t just tell you what could happen; it tells you whether doing something about it—extending therapy—will likely help.
That predictive edge is what makes BCI so powerful in this very specific clinical scenario. While the other tests dominate earlier in the cancer journey, BCI is about what happens after survival has been established, after the first round of battles has been won. It’s the voice that helps you determine how much longer you need to stay armored.
You might be asking, If these other tests are already widely used, why not just use one of them again at year five? It’s a reasonable thought—but unfortunately, these tests aren’t validated for decisions about extended hormone therapy. The way they calculate risk and benefit is tailored to the early phase of treatment, and their data doesn’t extend far enough into the years where BCI specializes. Re-using them at year five wouldn’t give you the right kind of insight—it’d be like trying to use a compass to measure altitude. Wrong tool, wrong terrain.
And if you’re wondering about cost and coverage—most insurance plans, including Medicare, treat BCI separately from the earlier genomic tests. It’s not seen as redundant, because it’s applied at a unique decision point and has clinical utility that’s been well-supported in peer-reviewed studies and national guidelines.
Ultimately, if you’ve already had a test like Oncotype DX in the past, it doesn’t make BCI unnecessary. In fact, many women have both—Oncotype at diagnosis, BCI at year five. They work in concert, not in competition.
Part nical Evidence and Validation Studies
So far, we’ve covered what the Breast Cancer Index (BCI) test is, what it measures, how it’s used, and where it fits compared to other genomic tools. But now we need to address what is arguably the most important question of all: Does it work? After all, the world of cancer care is full of promising ideas that never translate into meaningful change. So how do we know that BCI actually improves decision-making—or more crucially, patient outcomes?
The answer lies in clinical evidence, and there’s more of it than you might think. BCI isn’t just an experimental tool; it’s been through the rigors of scientific validation and peer-reviewed scrutiny. If you’re the kind of person who wants more than just a doctor’s recommendation—if you need to see the data to believe the benefit—this section is for you.
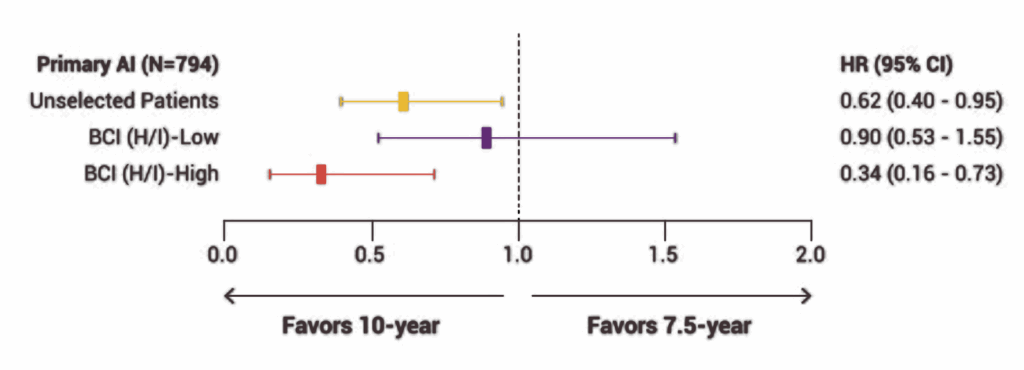
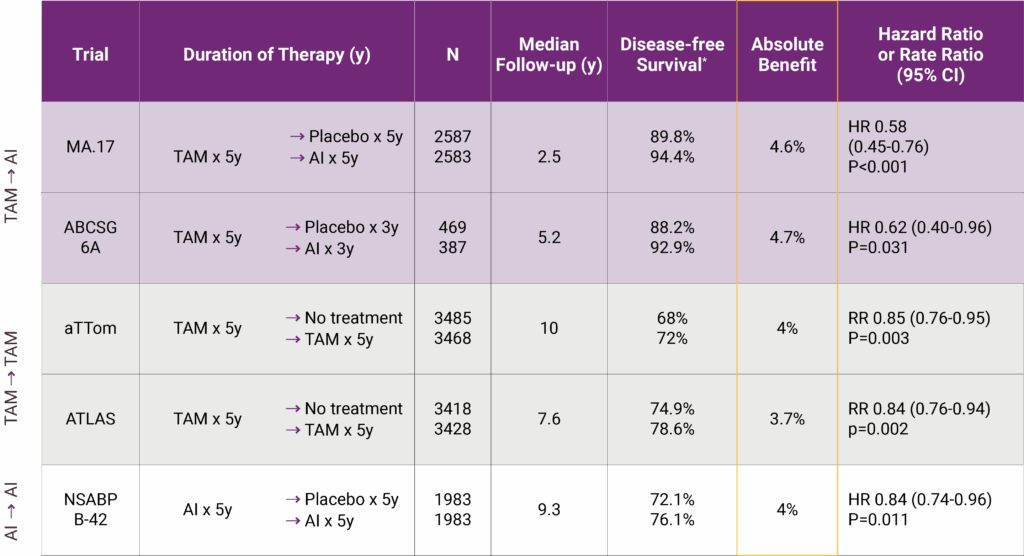
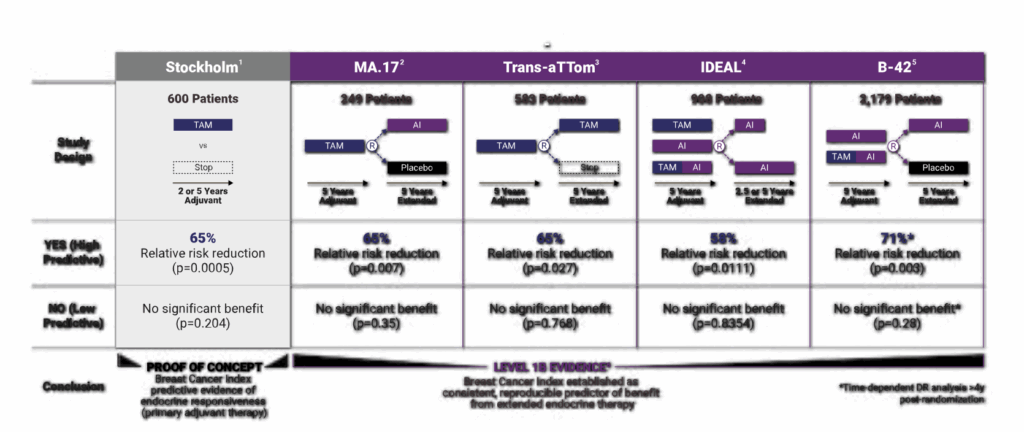
Let’s start with how BCI was validated. One of the key strengths of this test is that it was built and refined using large retrospective studies involving patients with hormone receptor-positive, early-stage breast cancer—specifically those who were postmenopausal and had completed at least five years of endocrine therapy. Researchers looked at tumor samples from these patients, ran BCI on the archived tissue, and then compared the results to real-world outcomes. They asked: did the risk score actually correlate with late recurrence? Did the H/I ratio accurately predict who benefited from extended endocrine therapy? In study after study, the answer was yes.
One landmark paper published in The Lancet Oncology analyzed data from the TransATAC trial, which had followed thousands of women treated with tamoxifen or anastrozole. In that study, BCI not only identified which patients were at low vs. high risk for late distant recurrence—it also revealed which women were likely to derive real benefit from five more years of therapy. That predictive component is what made the oncology world take notice. It was a moment of, “Wait, we can actually tell who needs more treatment and who doesn’t?”
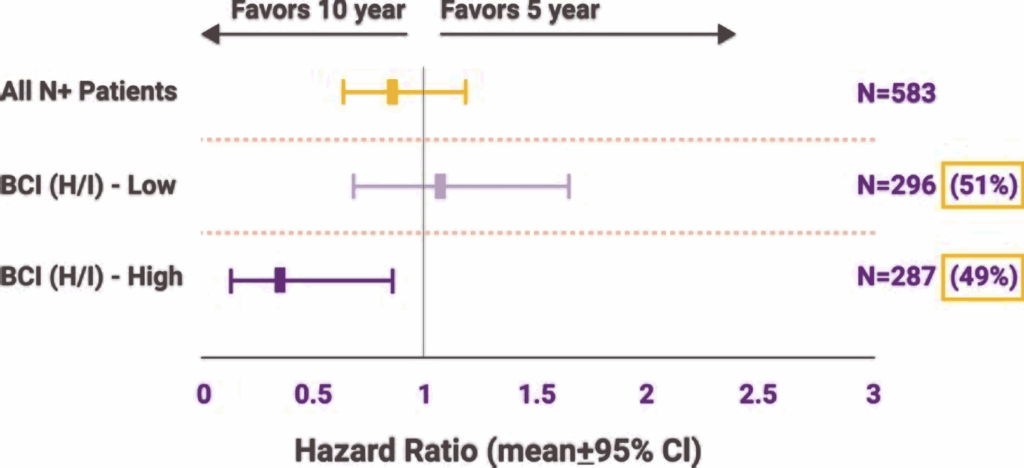
Another study, published in JAMA Oncology, looked specifically at node-positive patients—those with 1–3 positive lymph nodes, a group traditionally seen as higher risk. The findings? Even within that group, BCI could distinguish between those who might safely forgo extended therapy and those for whom the extra years made a statistically meaningful difference. That’s no small feat. Historically, if you had positive nodes, the assumption was always to treat more aggressively. BCI disrupted that paradigm by showing that not all node-positive tumors behave the same way at the genomic level.
And what about real-world impact? Several follow-up analyses have shown that BCI testing actually changes treatment decisions in practice. In one large community-based study, over 40% of patients who underwent BCI testing changed their treatment plans based on the results—either choosing to stop or continue endocrine therapy in ways they wouldn’t have without the test. That’s not a trivial stat. It means that this isn’t just a fancy lab tool collecting dust; it’s reshaping the choices people make every day.
You might be wondering: What do the national guidelines say? Is BCI officially endorsed? Yes—and increasingly so. Organizations like the National Comprehensive Cancer Network (NCCN) and the American Society of Clinical Oncology (ASCO) have incorporated BCI into their guidelines for extended endocrine therapy decisions. While it’s not mandatory (no test is), its inclusion in these guidelines reflects a growing consensus among experts that BCI adds genuine clinical value.
Of course, no test is infallible, and BCI is not immune to the usual limitations of retrospective study design and population bias. Some critics argue we still need more long-term, prospective validation in broader patient groups, especially premenopausal women or those with more complex treatment histories. That’s a fair point. But within its validated niche—postmenopausal, HR-positive, HER2-negative patients facing the extended therapy decision—the evidence supporting BCI is robust, consistent, and growing.
What’s perhaps most compelling isn’t just that the test works, but how it changes the doctor-patient conversation. Before BCI, the conversation around year five was often a vague blend of statistics and guesswork. Now, oncologists can say, “Here’s your personalized risk of recurrence. And here’s whether more treatment will likely help.” That shift—from generalized caution to individualized insight—can’t be overstated.
If you’re tracking evolving medical opinions, Miami Breast Cancer Conference 2025 covers how tools like BCI are influencing treatment timelines.
In the next section, we’ll get practical. What does BCI cost? Is it covered by insurance? Are there programs that help make it more accessible? Because while information is powerful, access is everything—and a test this useful should never be out of reach simply because of logistics or paperwork. Let’s demystify the real-world experience of getting the BCI.
Part 7: Clinical Evidence and Validation Studies
So far, we’ve covered what the Breast Cancer Index (BCI) test is, what it measures, how it’s used, and where it fits compared to other genomic tools. But now we need to address what is arguably the most important question of all: Does it work? After all, the world of cancer care is full of promising ideas that never translate into meaningful change. So how do we know that BCI actually improves decision-making—or more crucially, patient outcomes?
The answer lies in clinical evidence, and there’s more of it than you might think. BCI isn’t just an experimental tool; it’s been through the rigors of scientific validation and peer-reviewed scrutiny. If you’re the kind of person who wants more than just a doctor’s recommendation—if you need to see the data to believe the benefit—this section is for you.
Let’s start with how BCI was validated. One of the key strengths of this test is that it was built and refined using large retrospective studies involving patients with hormone receptor-positive, early-stage breast cancer—specifically those who were postmenopausal and had completed at least five years of endocrine therapy. Researchers looked at tumor samples from these patients, ran BCI on the archived tissue, and then compared the results to real-world outcomes. They asked: did the risk score actually correlate with late recurrence? Did the H/I ratio accurately predict who benefited from extended endocrine therapy? In study after study, the answer was yes.
One landmark paper published in The Lancet Oncology analyzed data from the TransATAC trial, which had followed thousands of women treated with tamoxifen or anastrozole. In that study, BCI not only identified which patients were at low vs. high risk for late distant recurrence—it also revealed which women were likely to derive real benefit from five more years of therapy. That predictive component is what made the oncology world take notice. It was a moment of, “Wait, we can actually tell who needs more treatment and who doesn’t?”
Another study, published in JAMA Oncology, looked specifically at node-positive patients—those with 1–3 positive lymph nodes, a group traditionally seen as higher risk. The findings? Even within that group, BCI could distinguish between those who might safely forgo extended therapy and those for whom the extra years made a statistically meaningful difference. That’s no small feat. Historically, if you had positive nodes, the assumption was always to treat more aggressively. BCI disrupted that paradigm by showing that not all node-positive tumors behave the same way at the genomic level.
And what about real-world impact? Several follow-up analyses have shown that BCI testing actually changes treatment decisions in practice. In one large community-based study, over 40% of patients who underwent BCI testing changed their treatment plans based on the results—either choosing to stop or continue endocrine therapy in ways they wouldn’t have without the test. That’s not a trivial stat. It means that this isn’t just a fancy lab tool collecting dust; it’s reshaping the choices people make every day.
You might be wondering: What do the national guidelines say? Is BCI officially endorsed? Yes—and increasingly so. Organizations like the National Comprehensive Cancer Network (NCCN) and the American Society of Clinical Oncology (ASCO) have incorporated BCI into their guidelines for extended endocrine therapy decisions. While it’s not mandatory (no test is), its inclusion in these guidelines reflects a growing consensus among experts that BCI adds genuine clinical value.
Of course, no test is infallible, and BCI is not immune to the usual limitations of retrospective study design and population bias. Some critics argue we still need more long-term, prospective validation in broader patient groups, especially premenopausal women or those with more complex treatment histories. That’s a fair point. But within its validated niche—postmenopausal, HR-positive, HER2-negative patients facing the extended therapy decision—the evidence supporting BCI is robust, consistent, and growing.
What’s perhaps most compelling isn’t just that the test works, but how it changes the doctor-patient conversation. Before BCI, the conversation around year five was often a vague blend of statistics and guesswork. Now, oncologists can say, “Here’s your personalized risk of recurrence. And here’s whether more treatment will likely help.” That shift—from generalized caution to individualized insight—can’t be overstated.
Cost, Insurance, and Accessibility
By now, you’re likely thinking: “Okay, this test sounds incredibly useful—possibly even pivotal—for deciding whether I need more endocrine therapy. But how much does it cost? Is it covered by insurance? And what if I can’t afford it out-of-pocket?” These are not minor questions. In fact, for many patients, they are the questions—the difference between benefitting from a powerful genomic tool and simply going without it.
Let’s begin with the basics: yes, the Breast Cancer Index test is covered by Medicare in the United States for eligible patients. That’s a significant endorsement, and it reflects the fact that BCI is not experimental or fringe—it has enough evidence behind it to meet Medicare’s standards for medical necessity. Specifically, Medicare will usually cover BCI for postmenopausal women with early-stage, HR-positive, HER2-negative breast cancer who are considering extended endocrine therapy. If you meet those criteria, you’re very likely to qualify for full or near-full coverage.
But what about private insurance? Fortunately, many private insurers—especially the larger ones—also recognize BCI as a medically necessary test. That said, coverage policies can vary widely depending on the plan, the insurer, and even the region. Some insurers may require prior authorization. Others may cover it automatically once a physician orders it and provides documentation supporting its use. It’s not a perfect system, but more often than not, patients who meet the clinical criteria do get approval.
Now, you might be wondering, What does this test actually cost if I had to pay for it myself? The list price of the Breast Cancer Index test has been estimated in the ballpark of $3,000 to $4,000, though the exact figure depends on the provider and setting. That’s a substantial number—but very few patients end up paying that full amount. Why? Because the lab that administers the BCI (usually Biotheranostics, a Hologic company) has a financial assistance program for patients who are uninsured, underinsured, or otherwise unable to afford the cost. They’ll often work directly with your insurance company on appeals, and if you still can’t get coverage, they’ll offer a reduced rate based on your financial circumstances.
This is where it’s worth pausing and saying: Don’t let a scary number on paper keep you from asking about the test. Many patients assume that genomic testing is out of reach, only to find that once the paperwork dust settles, the actual cost is far more manageable—or completely covered. It’s also worth remembering that this isn’t a repeat test; it’s a one-time decision point. You’re not signing up for years of payments or ongoing labs. You’re investing in a single, critical moment of decision-making that could shape your next five years of treatment and quality of life.
For those navigating this process, the practical steps usually look like this: Your oncologist submits the order, the testing company checks insurance coverage and handles prior authorization if needed, and you’re notified before the test is processed if there’s any out-of-pocket responsibility. If the quoted cost is too high, you can apply for financial support. It’s a little bureaucratic, yes—but the companies involved have built these systems knowing that patient access is a priority. This isn’t just about getting results; it’s about getting them ethically and equitably.
Still, it’s entirely fair to ask: Should this even be something I have to worry about? Shouldn’t life-saving, quality-of-life-preserving medical insights be part of routine care without a price tag attached? In an ideal system, yes. But we’re not there yet. Until we are, your best approach is to be proactive. If you think you qualify for BCI, talk to your oncologist, ask about coverage, and don’t hesitate to engage with the testing company directly. They have teams designed to navigate this with you.
One last note: coverage and availability may vary internationally. In some countries, BCI is not yet reimbursed or widely available. That doesn’t mean it’s inaccessible, but it might require going through private channels or advocating through your healthcare system. If you’re outside the U.S., your best bet is to speak with your oncologist and explore whether sending tissue abroad for testing is an option—something more patients are doing as genomic tools become globally relevant.
Up next, we’re going to pivot toward the human side of all this: how the Breast Cancer Index influences the patient experience. Because while it’s backed by data and insurance codes, its real power lies in helping you live more confidently, more comfortably, and more informed. And that’s not a number—it’s a life.
Patient Perspectives and Shared Decision-Making
If you’ve made it this far, it’s probably because you’re not just reading to gather facts—you’re reading to make a decision, or help someone else make one. And that’s the heart of what the Breast Cancer Index (BCI) test is really about. It’s not just a laboratory report, not just a score on a page—it’s a tool designed to help real people make deeply personal, often difficult decisions. So let’s talk about what that process actually feels like from the patient’s perspective.
Picture this: You’ve been on endocrine therapy for five years. You’re tired. The side effects have crept up on you. Maybe they’re manageable—maybe not. You’ve gotten used to them, but you certainly haven’t made peace with them. And just when you think you’re finally done, your oncologist says, “We need to talk about going another five.”
This is where patients often feel the weight of uncertainty. “What if I stop too soon?” “What if I keep going for no reason?” “What if this fatigue isn’t just annoying—but completely avoidable?” That’s where the BCI test enters—not to eliminate the uncertainty entirely, but to shrink it, to give you a compass in what often feels like a foggy forest.
One of the most powerful aspects of the BCI is the sense of personalization it offers. You’re no longer making choices based on generalized statistics or what happened to the last hundred patients in a trial. You’re looking at your own tumor’s biology—its unique genomic footprint—and asking, “What is this cancer likely to do? And what will actually make a difference for me?”
For many patients, this is profoundly empowering. Especially for those who feel whiplashed by the one-size-fits-all nature of cancer treatment, BCI provides a shift toward individualized care. Instead of the default recommendation being, “Let’s keep going, just to be safe,” you and your oncologist can actually discuss risk and benefit in the context of your specific biology, your values, and your quality of life.
And let’s talk about quality of life. That phrase gets tossed around a lot, but here it means something tangible. Extended endocrine therapy isn’t always gentle. Some patients tolerate it well, but others grapple with persistent joint pain, sleep disturbances, vaginal dryness, bone loss, and the slow, grinding erosion of energy and libido. These aren’t superficial inconveniences—they’re aspects of life that define how you feel in your body, your relationships, your daily peace of mind.
So imagine what it feels like to be told, after enduring all that, “You’re unlikely to benefit from continuing.” For some, it’s like being handed a key to freedom. It validates the fatigue, the emotional toll, the sense of dragging oneself through each day. For others, the decision might still be fraught—especially if they’re highly risk-averse—but now, at least, it’s informed by more than guesswork. It’s a choice backed by biology, not just fear.
On the flip side, if your BCI result suggests that extended therapy could provide real benefit, it can reframe the burden. You might still dread the next five years, but you understand why they matter. You’re not suffering blindly—you’re taking active steps to reduce your risk of a distant recurrence. That knowledge can turn frustration into purpose.
What’s equally important is that BCI shifts the dynamic between patient and clinician. This is shared decision-making at its best. The test doesn’t dictate what to do—it enhances the conversation. Your doctor brings the clinical context, you bring your lived experience, and the BCI brings objective, personalized insight to the table. Together, those three elements create a space where treatment decisions feel less like mandates and more like meaningful, collaborative steps.
This matters deeply because many patients—especially women—spend years in the passenger seat of their cancer care, following instructions, trusting the plan. BCI offers a rare moment to take the wheel. Not because you’re abandoning medical guidance, but because you’re equipped with the kind of information that puts you in command of your own trajectory.
It’s also worth noting that while BCI is most commonly discussed between patients and oncologists, other voices often enter the room: spouses, adult children, close friends. The test can bring clarity to those conversations too. Instead of family members saying, “Are you sure it’s okay to stop?” or “Why are you putting yourself through this if you don’t have to?”—now there’s an evidence-based rationale everyone can anchor to.
Frequently Asked Questions (FAQs)
1. What sets the Breast Cancer Index (BCI) test apart from other genomic tests?
BCI stands out because it specifically predicts whether extended endocrine therapy — beyond the standard five years — is likely to help. It provides two insights: your long-term risk of cancer recurrence (prognostic) and whether continued hormonal treatment is likely to benefit you (predictive). Other tests tend to focus more on chemotherapy decisions or early recurrence.
2. Who is the BCI test designed for, and does it work for all women?
BCI is currently validated for postmenopausal women with hormone receptor-positive, HER2-negative breast cancer. Research is ongoing for premenopausal use, so if you’re not yet postmenopausal, talk with your oncologist to weigh your options based on the latest data.
3. How is the test performed, and will I need a new biopsy?
No new biopsy is needed. BCI uses tumor tissue from your original surgery, typically stored in your hospital’s pathology department. This makes the test low-risk and easy to incorporate into follow-up care.
4. How quickly will I get my BCI results, and does insurance cover it?
Results are usually available within 10 to 14 days after the lab receives your tissue sample. Many U.S. insurance plans, including Medicare, cover the test for eligible patients. If not, financial support may be available through the testing company.
5. Can the results change over time or become outdated?
The test reflects the biology of your original tumor, which doesn’t change — so results remain valid over time. However, your personal health status and treatment tolerance do evolve, so decisions should be revisited with your oncologist in light of your current situation.
6. What should I do with my results — especially if extended therapy isn’t recommended?
Use BCI results as part of a shared decision-making process with your care team. If the test suggests no meaningful benefit from extended therapy, you may safely choose to stop at five years — potentially avoiding years of unnecessary side effects without compromising outcomes.
Closing Thoughts
The Breast Cancer Index test represents a leap forward in personalized breast cancer care. It transforms the challenging question of whether to continue endocrine therapy after five years into a data-driven, individualized decision. By tapping into the unique biology of your tumor, BCI helps you and your care team weigh the benefits and burdens of extended treatment with clarity and confidence.
No one wants to endure years of therapy without knowing if it truly matters. With BCI, that uncertainty diminishes. It empowers you to take ownership of your treatment journey, balancing scientific insight with your values and quality of life.
As medicine continues to evolve, tools like the Breast Cancer Index test exemplify how precision oncology isn’t just about surviving cancer—it’s about living well beyond it, on your terms. Whether you’re just starting to explore this option or already facing the decision of extended therapy, understanding BCI equips you with knowledge—a powerful ally in the fight against breast cancer.










