Biochemical Relapse in Prostate Cancer: Understanding PSA Recurrence and Management
- What Is Biochemical Relapse and Why It Matters
- PSA Thresholds and Definitions of Biochemical Recurrence
- Key Risk Factors for Biochemical Relapse After Treatment
- Imaging and Detection Strategies in Biochemical Relapse
- PSA Doubling Time: A Key Indicator of Recurrence Aggressiveness
- Salvage Radiation Therapy: When and How to Use It
- Hormone Therapy in the Context of Biochemical Recurrence
- Monitoring Strategies and the Role of Active Surveillance After BCR
- Role of Genomic and Molecular Testing After Biochemical Recurrence
- Intermittent Versus Continuous Androgen Deprivation Therapy
- Comparison of Management Options After Biochemical Recurrence
- Patient Communication and Emotional Impact of Biochemical Relapse
- Clinical Trials and Emerging Therapies for PSA Recurrence
- Long-Term Prognosis Following Biochemical Relapse
- Integrating Lifestyle and Comorbidity Management
- Guidelines and Recommendations for Clinicians
- Managing Biochemical Relapse with Clarity and Confidence
- Frequently Asked Questions (FAQ)
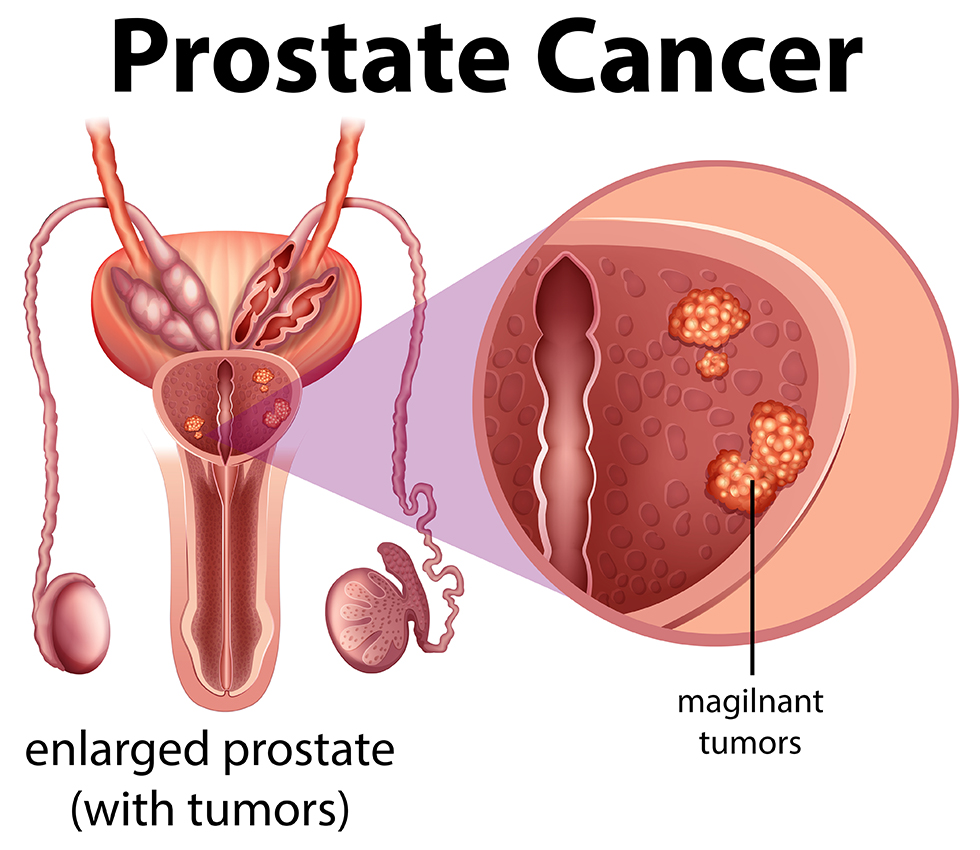
What Is Biochemical Relapse and Why It Matters
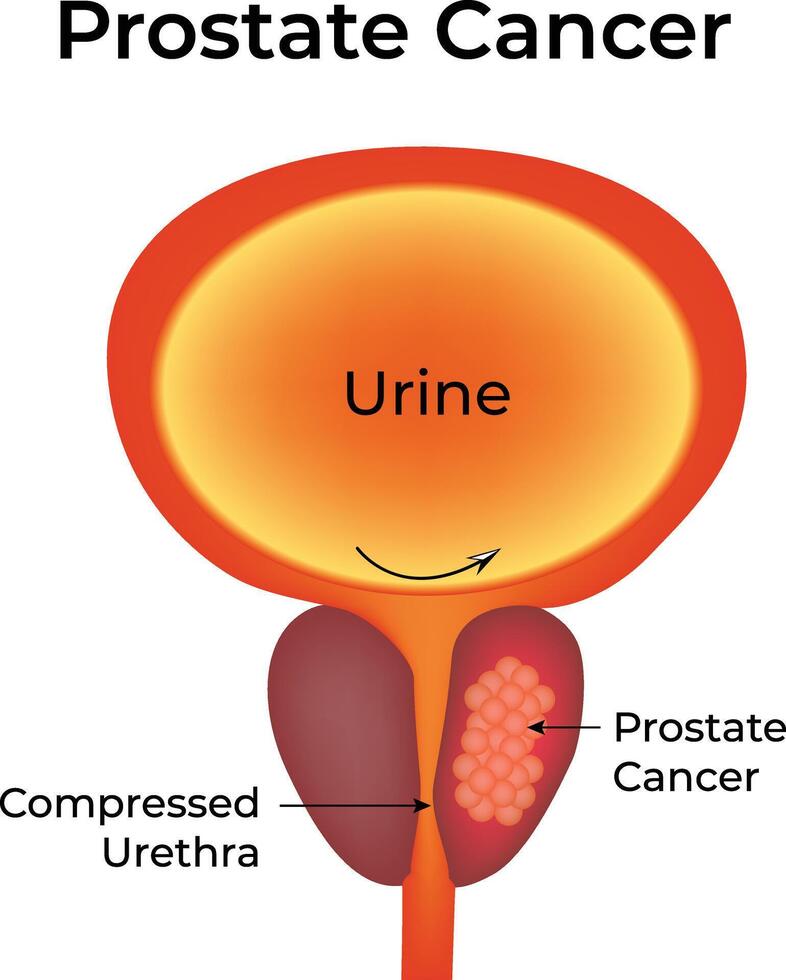
Biochemical relapse (BCR), also known as biochemical recurrence, refers to a rise in prostate-specific antigen (PSA) levels after definitive treatment for prostate cancer, such as surgery or radiation therapy. This rise occurs in the absence of visible disease on imaging, meaning the cancer may have returned or persisted at a microscopic level.
BCR is not a direct indicator of clinical symptoms or metastasis, but it plays a crucial role in the long-term monitoring of prostate cancer patients. Detecting PSA elevation early allows clinicians to decide whether to initiate salvage therapy or continue observation. Understanding the nuances of BCR helps patients and providers navigate next steps in a tailored and evidence-based manner.
PSA Thresholds and Definitions of Biochemical Recurrence
The definition of biochemical recurrence varies depending on the type of primary treatment a patient has received. After radical prostatectomy, BCR is typically defined as a confirmed PSA level of ≥0.2 ng/mL, with a second measurement verifying the rise. For patients treated with radiation therapy, the ASTRO (American Society for Therapeutic Radiology and Oncology) definition is a PSA increase of 2.0 ng/mL above the nadir.
These definitions matter because they guide decisions about salvage treatment and enrollment in clinical trials. A transient rise in PSA—known as a “PSA bounce”—is common after radiation and may not indicate true recurrence, further complicating the clinical picture.
Regular PSA testing post-treatment is essential. It provides a biochemical map of disease behavior, allowing clinicians to distinguish between harmless fluctuations and signs of aggressive recurrence.
Key Risk Factors for Biochemical Relapse After Treatment
Not all patients with prostate cancer face the same risk of biochemical recurrence. Several factors increase the likelihood of BCR, including:
- A high Gleason score at diagnosis
- Positive surgical margins
- Seminal vesicle invasion
- Lymph node involvement
- Rapid PSA doubling time after treatment
These clinical features often correlate with a more aggressive cancer phenotype, which is more likely to recur even after curative-intent therapy. For example, patients with a Gleason score of 8–10 are far more likely to experience BCR within five years of surgery than those with a Gleason 6.
Understanding these risk factors helps stratify patients into low, intermediate, and high-risk groups, guiding the frequency of follow-up testing and informing decisions about early salvage interventions.
Imaging and Detection Strategies in Biochemical Relapse
When a biochemical relapse occurs, the next step is often to determine whether the recurrence is local or systemic. However, conventional imaging methods such as CT and bone scans are often unable to detect microscopic disease. In recent years, advanced modalities like PSMA PET/CT and choline PET scans have become increasingly important.
These imaging technologies can detect small volumes of cancer even at low PSA levels, making them invaluable tools in early recurrence. However, accessibility and insurance coverage can be barriers in some regions.
This diagnostic challenge is similar to what we see in rare cancers with subtle presentations, such as basal cell carcinoma of the eyelid, where early detection depends on high suspicion and precise imaging interpretation.
PSA Doubling Time: A Key Indicator of Recurrence Aggressiveness
PSA doubling time (PSADT) is the period over which a patient’s PSA level doubles, typically measured in months. This metric has emerged as one of the most powerful predictors of clinical outcomes following biochemical relapse. A shorter PSADT (less than 6 months) often signals a more aggressive recurrence and a higher likelihood of developing distant metastases.
In contrast, a longer PSADT (over 12 months) may suggest a slower-growing or indolent recurrence, allowing for a more conservative or observational management approach. Clinicians routinely calculate PSADT using at least three PSA readings over six months to obtain reliable results.
PSADT guides timing for imaging, the need for salvage therapy, and consideration of systemic treatment. It’s particularly useful in distinguishing patients who may benefit from early intervention from those who can be safely monitored.
Salvage Radiation Therapy: When and How to Use It
For patients who underwent radical prostatectomy, the most common post-BCR treatment is salvage radiation therapy (SRT). SRT is typically administered to the prostate bed and, in some cases, to nearby lymph nodes. It is most effective when initiated at low PSA levels, generally before the PSA exceeds 0.5–1.0 ng/mL.
Studies suggest that early initiation of SRT in eligible patients can delay or prevent the progression to metastasis. However, the success of SRT depends heavily on prior pathology, surgical margin status, and PSA kinetics.
Just as coding precision is essential for representing tumor recurrence in biliary tract cancers, like those described in the ICD-10 classification for bile duct cancer, accurate staging and treatment response documentation matter profoundly in prostate cancer as well. Bile duct cancer icd 10 – in the context of coding and choice of therapy for relapses.
Hormone Therapy in the Context of Biochemical Recurrence
Androgen deprivation therapy (ADT), often referred to as hormone therapy, can be used as a salvage approach for patients with high-risk biochemical relapse. It reduces circulating testosterone, thereby slowing cancer growth. However, ADT is typically reserved for:
- Patients with rapid PSA doubling time
- High initial Gleason scores
- Evidence of metastasis or strong suspicion thereof
ADT is not without side effects. Patients may experience fatigue, decreased libido, hot flashes, and metabolic changes. As such, the decision to initiate hormone therapy during BCR is individualized, balancing potential benefit with quality-of-life considerations.
There is growing interest in intermittent ADT protocols, where therapy is paused and resumed based on PSA levels, potentially reducing long-term side effects without compromising outcomes.
Monitoring Strategies and the Role of Active Surveillance After BCR
Not all biochemical relapses require immediate intervention. In many cases, especially those with slow PSA kinetics, active surveillance is a valid strategy. This approach involves:
- Regular PSA testing every 3–6 months
- Periodic imaging (e.g., MRI or PET scans)
- Close clinical follow-up
Active surveillance avoids overtreatment and allows patients to maintain quality of life until clear signs of disease progression emerge. The decision to surveil versus treat is often based on PSA doubling time, patient age, comorbidities, and individual preference.
Similar principles apply to other indolent cancers, such as basal cell carcinoma of the skin, where observation may be appropriate for slow-growing lesions in non-critical areas. Basal cell skin cancer icd 10 – in the context of active surveillance in slowly progressing tumors
Role of Genomic and Molecular Testing After Biochemical Recurrence
As prostate cancer management becomes increasingly personalized, genomic classifiers and molecular assays have emerged as powerful tools in evaluating biochemical relapse. Tests such as Decipher, Oncotype DX Genomic Prostate Score, and Prolaris assess the tumor’s biological aggressiveness and help predict the likelihood of metastasis after BCR.
These tests can inform:
- Whether salvage therapy is warranted
- The potential benefit of adding ADT to radiation
- Prognosis in the absence of visible disease
Genomic testing is especially helpful when traditional markers (Gleason score, PSA, margins) offer unclear guidance. Molecular profiles provide an extra layer of decision support in an otherwise gray zone of recurrence management.
Intermittent Versus Continuous Androgen Deprivation Therapy
In patients undergoing hormone therapy for biochemical relapse, there are two general approaches: continuous ADT and intermittent ADT (iADT). The latter involves giving treatment until PSA drops to a low threshold, then pausing therapy until PSA rises again.
Research suggests that iADT can offer similar cancer control compared to continuous ADT, with fewer side effects such as sexual dysfunction, weight gain, and bone loss. It may also delay the onset of resistance to hormone therapy.
Choosing between the two requires evaluating:
- PSA kinetics
- Patient preference
- Comorbidities
- Risk of future metastasis
This strategy reflects a growing trend in oncology of reducing overtreatment and improving survivorship outcomes without compromising disease control.
Comparison of Management Options After Biochemical Recurrence
| Treatment Option | Indicated For | Advantages | Limitations |
| Salvage Radiation | Post-prostatectomy with local recurrence | Potential cure if early; non-systemic | Less effective with high PSA |
| Androgen Deprivation | High-risk or metastatic recurrence | Rapid PSA reduction; systemic control | Side effects; not curative |
| Intermittent ADT | Biochemical recurrence without visible disease | Quality of life preservation | Long-term efficacy still under study |
| Active Surveillance | Slow PSA doubling time, elderly patients | Avoids overtreatment | Risk of missing metastatic progression |
| Genomic Testing | Ambiguous risk profile | Personalized prognosis | Cost, availability |
This comparative overview helps clinicians and patients make informed choices that align with the biology of the recurrence and the goals of care.
Patient Communication and Emotional Impact of Biochemical Relapse
While biochemical relapse is often asymptomatic, the psychological impact can be profound. Patients may experience renewed anxiety, fear of progression, and uncertainty about what the rising PSA truly means. For many, BCR feels like “the cancer is back,” even when imaging is negative.
Clear communication is essential. Physicians should explain the meaning of BCR, available options, and the fact that many patients live for years without clinical progression after PSA recurrence. Providing written information, support groups, and referrals to psycho-oncology services can help mitigate distress.
Framing the situation as “biological activity” rather than “disease relapse” can also shift the emotional tone and empower patients to stay engaged in their monitoring and treatment plans.
Clinical Trials and Emerging Therapies for PSA Recurrence
Biochemical relapse marks a unique window where prostate cancer has become biochemically active again, yet has not produced clinical or radiographic evidence of spread. This phase is ideal for exploring novel interventions through clinical trials. Trials provide access to cutting-edge treatments that are still under investigation but have shown promise in early studies.
Ongoing research includes:
- Second-generation hormonal agents, such as enzalutamide or apalutamide, that block androgen signaling more effectively than standard therapies.
- Radioligand therapy, particularly those targeting prostate-specific membrane antigen (PSMA), which delivers targeted radioactive isotopes to prostate cancer cells.
- Immunotherapy trials, testing agents like checkpoint inhibitors, which may enhance the body’s immune response against cancer.
- DNA repair–targeting agents, including PARP inhibitors, for patients with BRCA or ATM mutations, representing a more tailored approach to therapy.
Participation in trials is not only an opportunity for the individual but also contributes to the future of prostate cancer care. Early BCR is an important enrollment criterion in many trials, and patients who meet it should consider asking their oncologist about eligibility.
Long-Term Prognosis Following Biochemical Relapse
The long-term outcome after biochemical relapse can vary greatly from person to person. Several factors influence the prognosis:
- PSA doubling time (shorter doubling time predicts faster progression)
- Gleason score and pathological features at diagnosis
- Time to relapse (an earlier BCR following primary treatment often signals more aggressive disease)
- Response to salvage therapies, such as radiation or ADT
Some patients with BCR may remain progression-free for over a decade with minimal or no treatment, especially when PSA rises slowly and other risk factors are favorable. Others, particularly those with aggressive cancer biology or fast PSA kinetics, may develop metastatic disease within a few years.
Importantly, BCR does not always require immediate intervention. Careful monitoring, combined with individualized risk assessment, allows patients to maintain a high quality of life while staying vigilant. When necessary, early treatment based on clear risk indicators can significantly improve long-term control and survival outcomes.
Integrating Lifestyle and Comorbidity Management
The diagnosis of biochemical recurrence should not be viewed in isolation from a patient’s overall health. Men with prostate cancer often live for years or even decades post-treatment, and the management of comorbidities becomes an essential part of survivorship.
Key lifestyle and health interventions include:
- Heart-healthy nutrition, reducing red meats and processed sugars while increasing intake of vegetables, lean proteins, and healthy fats.
- Regular physical activity, such as walking, resistance training, or swimming, to maintain bone density and muscle mass—especially important for those on ADT.
- Monitoring for metabolic changes, like insulin resistance or weight gain, which can be exacerbated by hormonal therapies.
- Mental health care, including support groups, stress reduction strategies, and access to psycho-oncology services to address anxiety and emotional distress.
These principles align with the approach used in other slow-progressing cancers, such as basal cell carcinoma of the eyelid, where a combination of conservative monitoring and systemic health optimization ensures better outcomes. Basal cell cancer eyelid to highlight the importance of a systems approach to slowly progressing tumors.
Guidelines and Recommendations for Clinicians
International guidelines provide a structured framework for managing biochemical relapse. Key organizations such as the National Comprehensive Cancer Network (NCCN), European Association of Urology (EAU), and American Society of Clinical Oncology (ASCO) offer regularly updated protocols based on evolving evidence.
According to these guidelines:
- PSA should be monitored every 3 to 6 months after primary treatment.
- Salvage radiation is recommended for eligible post-prostatectomy patients with low PSA and adverse pathology.
- ADT is reserved for high-risk recurrence or when metastasis is confirmed.
- Advanced imaging, especially PSMA PET/CT, should be considered even at low PSA levels if recurrence is suspected.
- Genomic profiling is encouraged when conventional risk stratification is inconclusive.
Adhering to these recommendations ensures that patients receive evidence-based, individualized care, and it also promotes consistency across institutions. Clinicians should remain up to date with changes, especially as new biomarkers, imaging tools, and therapies are integrated into standard practice.
Managing Biochemical Relapse with Clarity and Confidence
Biochemical relapse in prostate cancer represents a critical inflection point in a patient’s journey. While it often triggers emotional responses due to fears of recurrence, the rise in PSA does not always indicate imminent clinical progression. A clear understanding of risk factors, kinetics, and available options allows for rational, measured responses rather than reflexive interventions.
Today’s tools—ranging from PSMA PET scans to genomic classifiers and emerging therapies—equip clinicians and patients with more precision than ever before. Whether the decision is to monitor, to treat, or to explore novel trials, choices can now be based on tumor biology and patient preferences.
Ultimately, the goal in managing BCR is to maintain quality of life, prevent overtreatment, and intervene when the evidence truly supports benefit. With the right balance of vigilance and restraint, biochemical relapse becomes a manageable event—not a definitive setback.
Frequently Asked Questions (FAQ)
What is biochemical relapse in prostate cancer?
Biochemical relapse refers to a rise in PSA levels after treatment for prostate cancer, without visible evidence of disease on scans. It signals that cancer cells may still be present and active, but at a microscopic level.
How is biochemical recurrence diagnosed?
After prostatectomy, BCR is typically diagnosed when PSA reaches ≥0.2 ng/mL on two consecutive tests. After radiation therapy, it’s defined as a PSA rise of 2.0 ng/mL above the lowest recorded level (nadir).
Does a rising PSA always mean cancer is back?
Not necessarily. Some PSA rises can be temporary (like a “PSA bounce” after radiation) or due to benign conditions. However, persistent or steadily rising PSA usually indicates biochemical relapse.
What is PSA doubling time and why is it important?
PSA doubling time (PSADT) measures how quickly PSA levels increase. A short PSADT (under 6 months) may suggest aggressive recurrence, while a longer one (over 12 months) indicates slower progression.
Can imaging detect cancer during biochemical relapse?
Conventional imaging may not detect disease at early PSA levels. Advanced techniques like PSMA PET scans are more sensitive and can locate small cancer deposits not seen on CT or bone scans.
What are treatment options after BCR?
Options include salvage radiation therapy, androgen deprivation therapy, active surveillance, and participation in clinical trials. The choice depends on PSA trends, prior treatments, and risk factors.
Is salvage radiation effective after prostate surgery?
Yes, especially when PSA levels are still low. Salvage radiation can target remaining cancer in the prostate bed and may cure localized recurrence if administered early.
What are the side effects of hormone therapy for BCR?
Hormone therapy may cause fatigue, hot flashes, loss of libido, weight gain, and bone thinning. These side effects can impact quality of life, especially with long-term use.
Can you live a long time with biochemical relapse?
Many patients do. Some live for years without developing symptoms or metastases, especially if the PSA rises slowly. With careful monitoring, outcomes can remain favorable.
Is active surveillance safe after BCR?
Yes, for select patients with slow PSA doubling times and low overall risk. Active surveillance involves regular PSA checks and imaging without immediate treatment.
What is intermittent androgen deprivation therapy?
Intermittent ADT involves cycling hormone therapy on and off based on PSA levels. It may help preserve quality of life while controlling cancer in patients without rapid progression.
Do lifestyle changes affect PSA recurrence?
While they can’t prevent recurrence alone, healthy habits—like diet, exercise, and stress management—can improve overall health, reduce treatment side effects, and enhance resilience.
Can genomic tests help decide treatment after BCR?
Yes. Genomic profiling can predict the biological aggressiveness of recurrence and help guide decisions about whether to pursue active treatment or monitoring.
Should patients with BCR join clinical trials?
Participation in trials offers access to novel therapies and helps advance research. Patients with high-risk recurrence or uncertain treatment options may particularly benefit.
Is biochemical relapse the same as metastatic prostate cancer?
No. BCR is a biochemical event—it indicates PSA increase without confirmed spread. Metastatic cancer requires evidence of spread to bones, lymph nodes, or other organs.