Androstenedione, Aromatase Inhibitors, and Prostate Cancer: What Every Patient Should Know
- What Is Androstenedione and Why It Matters in Prostate Cancer
- How Aromatase Inhibitors Work in the Male Endocrine Environment
- The Hormonal Feedback Loop: Interactions Between Androstenedione, Estrogen, and Testosterone
- Clinical Use of Aromatase Inhibitors in Prostate Cancer: Current Evidence and Trials
- The Role of Estrogens in Male Prostate Tissue
- Key Comparisons of Aromatase Inhibitors Used in Male Hormonal Oncology
- Risks and Side Effects of Using Aromatase Inhibitors in Men
- Clinical Trials Involving Aromatase Inhibitors in Prostate Cancer
- Interaction Between Aromatase Inhibition and Immune Modulation
- Patient Selection Criteria for Aromatase Inhibitor Use
- Comparing Aromatase Inhibitors and Traditional ADT
- Understanding the Mechanism of Aromatase Inhibitors in Prostate Tissue
- Clinical Trials and Evidence for Aromatase Inhibitor Use in Prostate Cancer
- Comparative Overview: Androstenedione vs. Other Estrogen Precursors
- Patient Selection for Aromatase Inhibitor Therapy in Prostate Cancer
- Monitoring Treatment Response and Hormonal Shifts
- Potential Risks and Adverse Effects of Aromatase Inhibitor Use
- Research Gaps and Future Directions
- Integrating Aromatase Inhibitors into Broader Prostate Cancer Therapy
- Frequently Asked Questions (FAQ)
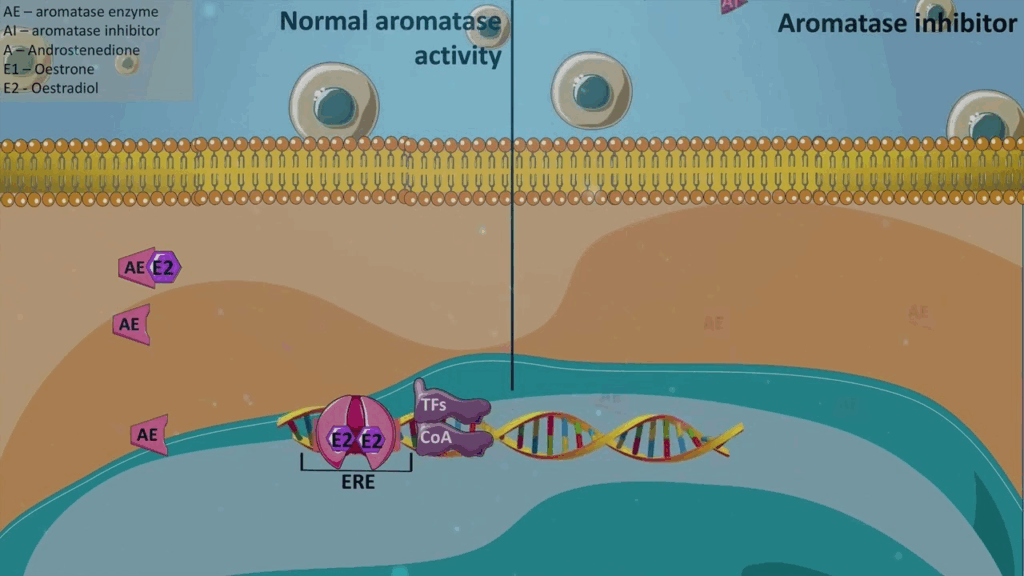
What Is Androstenedione and Why It Matters in Prostate Cancer
Androstenedione is a steroid hormone produced by the adrenal glands, testes, and ovaries. It serves as a precursor to both testosterone and estrogen, making it a central figure in the endocrine system. In the context of prostate cancer, androstenedione becomes highly relevant because prostate tumors often depend on androgens, particularly testosterone and dihydrotestosterone (DHT), for growth and proliferation. The presence of androstenedione in the bloodstream means that even when direct testosterone synthesis is suppressed—such as with androgen deprivation therapy (ADT)—prostate cancer cells may still access alternative sources of androgenic stimulation. Understanding how this precursor operates is crucial in targeting hormone-driven cancer progression and helps clinicians design better strategies for managing hormone-refractory prostate cancer.
How Aromatase Inhibitors Work in the Male Endocrine Environment
Aromatase is an enzyme that converts androstenedione and testosterone into estrogen, predominantly estrone and estradiol. In men, the aromatase enzyme is found in adipose tissue, liver, and even in the tumor microenvironment. When androstenedione levels are elevated, so too can be the local estrogen concentrations—something increasingly linked to the progression of hormone-sensitive cancers, including certain subtypes of prostate cancer. Aromatase inhibitors block this conversion pathway, effectively reducing estrogen levels in the body. While this is traditionally considered more relevant in breast cancer therapy, emerging studies suggest that suppressing estrogen may also slow down prostate tumor progression in select cases. Some research even points to estrogen as a contributor to prostate inflammation, which may worsen the prognosis. The impact of aromatase inhibition in men is still under investigation, but its significance continues to grow in clinical discussions.
The Hormonal Feedback Loop: Interactions Between Androstenedione, Estrogen, and Testosterone
The endocrine feedback system is a highly regulated loop in which the hypothalamus, pituitary gland, and gonads interact through hormones. Androstenedione’s conversion into testosterone and estrogens influences this loop in both direct and indirect ways. In the presence of aromatase inhibitors, there’s a shift in the hormonal balance—less estrogen is available, potentially altering luteinizing hormone (LH) and follicle-stimulating hormone (FSH) output. This hormonal reshuffling can either help or hinder prostate cancer management depending on the individual’s metabolic context and tumor behavior. For example, in obese patients, elevated aromatase activity often leads to higher estrogen levels, which may blunt the effects of ADT. Blocking aromatase in such cases might indirectly support better androgen suppression. However, clinicians must weigh the risk of affecting bone health and cardiovascular function, both of which are tied to estrogen levels in men.
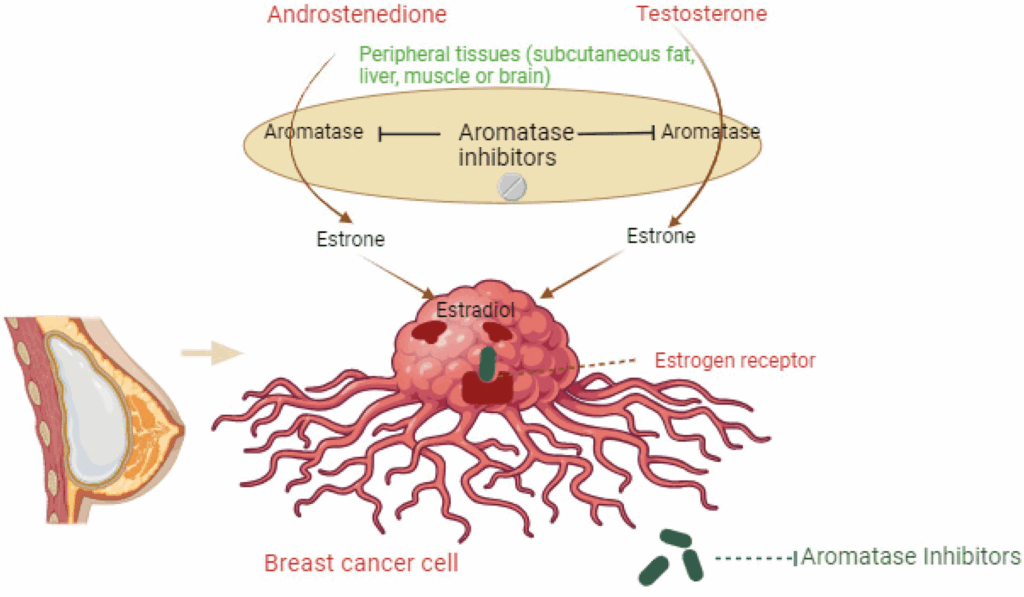
Clinical Use of Aromatase Inhibitors in Prostate Cancer: Current Evidence and Trials
Aromatase inhibitors are not yet standard treatment in prostate cancer, but several clinical trials and off-label studies have explored their use in androgen-independent or recurrent disease. Third-generation inhibitors like anastrozole, letrozole, and exemestane have been studied for their ability to delay biochemical progression or reduce PSA levels in patients not responding to first-line ADT. A phase II trial published in Clinical Cancer Research showed that letrozole was able to achieve modest PSA stabilization in patients with castration-resistant prostate cancer (CRPC). Another small-scale study suggested a benefit in combining aromatase inhibitors with antiandrogens like bicalutamide. Despite these findings, there remains no consensus on dosing, duration, or patient selection. This is partly due to the complex role of estrogens in male physiology—where complete estrogen suppression can lead to adverse outcomes like osteoporosis and lipid imbalances. More robust studies are needed to determine whether aromatase inhibitors can be integrated into broader prostate cancer protocols or remain reserved for experimental use.
The Role of Estrogens in Male Prostate Tissue
While androgens are the main drivers of prostate development and cancer progression, estrogens also play an essential, though often underestimated, role in male prostate physiology. Estrogens, derived either from direct synthesis or from the aromatization of androstenedione and testosterone, bind to estrogen receptors (ERα and ERβ) present in prostate tissue. ERα is typically associated with promoting inflammation and cellular proliferation, while ERβ may act as a tumor suppressor in certain contexts. This duality makes estrogen signaling complex and variable depending on the local receptor expression profile. Elevated estrogen levels—especially in aging men or those with increased body fat—can disrupt normal tissue regulation and contribute to malignant changes in the prostate. Understanding how estrogen functions locally has led researchers to explore the potential of estrogen-modulating therapies, including aromatase inhibitors, as part of a broader strategy to control tumor dynamics.
Androstenedione as a Biomarker in Prostate Cancer Risk Assessment
Recent studies have investigated the utility of androstenedione as a predictive biomarker for prostate cancer diagnosis and progression. Elevated serum androstenedione may indicate increased adrenal androgen production or reduced conversion efficiency to downstream androgens, signaling hormonal imbalances. Some researchers suggest that pre-treatment levels of androstenedione could help identify patients more likely to benefit from aromatase inhibition or adrenal-targeted therapies. However, variability in laboratory standards and patient metabolism complicates the interpretation of results. As part of a diagnostic panel, androstenedione might be more useful when combined with other markers such as testosterone, dihydrotestosterone, estradiol, and prostate-specific antigen (PSA). It’s important to note that unlike PSA, androstenedione is not prostate-specific, which limits its value as a standalone diagnostic tool but enhances its role in hormonal profiling and treatment stratification.
Key Comparisons of Aromatase Inhibitors Used in Male Hormonal Oncology
| Aromatase Inhibitor | Generation | Common Use | Relevance in Prostate Cancer | Notable Side Effects |
| Anastrozole | 3rd | Breast cancer (female) | Studied off-label in CRPC; modest PSA reductions | Joint pain, decreased bone density |
| Letrozole | 3rd | Breast cancer (female) | Some benefit in PSA stabilization; under trial | Hot flashes, fatigue |
| Exemestane | 3rd | Breast cancer (postmenopausal) | Irreversible inhibition may suit long-term androgen suppression | Nausea, risk of osteoporosis |
This table highlights the primary agents used in aromatase inhibition, though none are currently FDA-approved for prostate cancer specifically. Their application is generally investigational or off-label, often in conjunction with other hormone therapies or after first-line ADT failure.
Risks and Side Effects of Using Aromatase Inhibitors in Men
While aromatase inhibitors may offer potential benefits in select prostate cancer scenarios, they are not without risks. Estrogen plays a crucial role in maintaining bone health, cardiovascular integrity, cognitive function, and sexual health in men. Long-term suppression of estrogen using aromatase inhibitors has been linked to decreased bone mineral density, increasing the risk of fractures and osteoporosis—particularly concerning for older prostate cancer patients already undergoing androgen suppression. Cardiovascular events such as lipid imbalance and arterial stiffness have also been reported, especially when estrogen levels drop significantly. Additional side effects include joint stiffness, fatigue, and hot flashes—symptoms often underappreciated in male oncology. Due to these risks, clinicians must carefully assess whether the potential benefits of estrogen suppression outweigh the systemic consequences, and consider bone density monitoring, dietary support, and patient-specific comorbidities in therapy planning.
Clinical Trials Involving Aromatase Inhibitors in Prostate Cancer
Clinical investigations into the use of aromatase inhibitors in prostate cancer have yielded mixed but intriguing results. Several small-scale trials have explored drugs like anastrozole and letrozole, particularly in cases of castration-resistant prostate cancer (CRPC) where traditional androgen deprivation therapy (ADT) has failed. In these contexts, some patients experienced stabilization or slight reduction in prostate-specific antigen (PSA) levels, suggesting a possible role for estrogens in driving disease progression even after testosterone suppression. However, large-scale randomized controlled trials are lacking, and no aromatase inhibitor is currently FDA-approved for prostate cancer. These trials often include combination approaches with LHRH agonists or anti-androgens to assess synergistic effects. Importantly, researchers continue to explore biomarkers that might predict which patients would benefit most from such therapy, aiming to personalize hormonal strategies for better outcomes.
Interaction Between Aromatase Inhibition and Immune Modulation
Emerging research suggests that hormonal regulation via aromatase inhibition may also have downstream effects on immune system function in men with prostate cancer. Estrogens modulate immune responses in both innate and adaptive systems, and their reduction could impact cytokine signaling, T-cell activity, and tumor microenvironment behavior. For example, lower estrogen levels may decrease regulatory T-cell activity, potentially enhancing the immune system’s ability to target and destroy cancer cells. However, this effect can also introduce risks, such as increased systemic inflammation or autoimmune reactivity. The immunomodulatory consequences of aromatase inhibition in prostate cancer remain largely theoretical but are under investigation in preclinical models. This intersection is particularly relevant when considering combination therapies that involve immunotherapy agents like checkpoint inhibitors, where hormonal balance may influence therapeutic responsiveness.
Patient Selection Criteria for Aromatase Inhibitor Use
Given the absence of formal guidelines, selecting appropriate candidates for aromatase inhibitor therapy in prostate cancer requires a nuanced, individualized approach. Ideal candidates are typically those with advanced or treatment-resistant disease who have already undergone conventional ADT and show hormonal profiles suggestive of persistent estrogen influence. This might include elevated serum estradiol or androstenedione levels, obesity-related aromatization, or progression despite castrate testosterone levels. Additionally, patients with bone metastases may warrant closer scrutiny due to the bone-thinning effects of estrogen depletion. A multidisciplinary evaluation involving urologists, endocrinologists, and oncologists is essential to weigh the benefits and risks. Genetic profiling, body composition, and comorbidities also play crucial roles in this decision-making process. Given the experimental nature of this treatment path, patients should ideally be enrolled in clinical trials or monitored under expanded access protocols.
Comparing Aromatase Inhibitors and Traditional ADT
Traditional androgen deprivation therapy, often achieved via LHRH agonists or orchiectomy, remains the cornerstone of prostate cancer treatment. These approaches focus on reducing circulating testosterone to castrate levels. In contrast, aromatase inhibitors do not directly target testosterone but instead prevent its conversion to estrogen, which may act as an independent proliferative agent in some prostate tumors. While ADT is effective in most hormone-sensitive cases, it often leads to eventual resistance. Aromatase inhibitors may complement ADT by addressing estrogen-driven escape mechanisms. However, the side effect profiles differ: ADT commonly induces hot flashes, muscle wasting, and sexual dysfunction, while aromatase inhibitors present unique risks such as osteoporosis and altered lipid metabolism. Some patients may benefit from dual-pathway suppression, but this increases complexity and necessitates enhanced monitoring.
Understanding the Mechanism of Aromatase Inhibitors in Prostate Tissue
Aromatase inhibitors function by targeting the enzyme aromatase, which is responsible for converting androgens like androstenedione and testosterone into estrogens. While aromatase activity is more commonly associated with breast tissue and estrogen-related cancers, research has shown that aromatase also plays a functional role in prostate tissue, particularly in the tumor microenvironment. In prostate cancer, the local production of estrogens within the prostate can contribute to cancer cell proliferation, differentiation, and resistance to traditional androgen deprivation therapies (ADT). Aromatase inhibitors, therefore, represent a mechanism to further reduce estrogen influence in the prostate, complementing the androgen-blocking actions already used in prostate cancer management. Blocking this pathway is especially relevant in cases where tumors are found to express estrogen receptors (ERs), which may mediate cell signaling leading to tumor growth.
Clinical Trials and Evidence for Aromatase Inhibitor Use in Prostate Cancer
Several clinical trials have explored the off-label or investigational use of aromatase inhibitors in the context of prostate cancer. Although these drugs are not yet standard treatment, initial studies suggest a potential therapeutic benefit, particularly when used in combination with standard androgen deprivation therapy. Letrozole and anastrozole, two commonly studied agents, have been tested in early-phase trials for their effects on PSA levels, tumor progression, and hormonal markers in patients with hormone-refractory prostate cancer. Results show that some patients exhibit biochemical responses, such as reduced PSA levels or stabilization of disease. However, the evidence remains mixed, and larger trials are needed to determine optimal dosing, patient selection criteria, and long-term outcomes. The role of aromatase inhibition is still being defined, but ongoing research continues to suggest that these agents may hold value in specific subtypes of prostate cancer, especially where intratumoral estrogen signaling is robust.
Comparative Overview: Androstenedione vs. Other Estrogen Precursors
| Estrogen Precursor | Source in Males | Enzyme Pathway | Role in Prostate Cancer | Therapeutic Implication |
| Androstenedione | Adrenal glands, testes | Aromatase → Estrone | May fuel local estrogen production | Targeted by aromatase inhibitors |
| Testosterone | Testes | Aromatase → Estradiol | Converts to potent estrogen locally | Reduced by both ADT and aromatase inhibitors |
| Dehydroepiandrosterone (DHEA) | Adrenal cortex | Aromatase → Estrogens | Minor contributor but measurable | Limited data on inhibition effects |
| Estrone | Peripheral conversion | – | Weak estrogen with mitogenic effects | Less targeted, but accumulates post-aromatase activity |
| Estradiol | Conversion from testosterone | – | High estrogenic potency | Potentially a growth promoter in ER+ tumors |
This comparative view emphasizes why focusing on androstenedione, among other estrogen precursors, is critical in understanding hormonal influences on prostate cancer biology.
Patient Selection for Aromatase Inhibitor Therapy in Prostate Cancer
Selecting appropriate candidates for aromatase inhibitor therapy in prostate cancer is a nuanced process. While not every patient will benefit from this class of medication, those with specific tumor characteristics may experience measurable advantages. Men with hormone-refractory prostate cancer, particularly those who exhibit signs of estrogen receptor positivity or increased aromatase expression in tumor biopsies, are among the most likely to benefit. In addition, patients who have shown resistance or limited response to standard ADT may be evaluated for inclusion in clinical trials involving aromatase inhibitors. Genetic markers, circulating hormone profiles, and biopsy analysis can help oncologists determine suitability. Given the experimental nature of aromatase inhibition in prostate cancer, this approach is generally considered when conventional therapies fail or when there’s a strong biological rationale to address estrogen signaling specifically.
Monitoring Treatment Response and Hormonal Shifts
Once a patient begins therapy involving aromatase inhibition—especially in conjunction with other hormonal strategies—it becomes essential to closely monitor biochemical markers, clinical symptoms, and imaging data. The primary measurable indicator in prostate cancer is PSA (prostate-specific antigen), which can reflect tumor activity. However, when introducing aromatase inhibitors, additional hormone panels are needed, including serum estradiol, testosterone, and androstenedione levels, to evaluate whether estrogen suppression is occurring effectively. Physicians may also track circulating tumor cells (CTCs), inflammatory markers, or even advanced genomic profiles to gain further insight into the biological response. Clinically, a reduction in tumor burden symptoms—such as lower urinary tract symptoms, pain from metastasis, or general fatigue—may support a positive treatment outcome. It’s also vital to assess bone density regularly, since lower estrogen levels can predispose patients to osteoporosis, especially when combined with androgen deprivation therapy.
Potential Risks and Adverse Effects of Aromatase Inhibitor Use
Although aromatase inhibitors are generally well-tolerated in female breast cancer patients, their use in men—especially those with prostate cancer—presents distinct challenges. One of the most concerning risks is the development of bone mineral density loss, which can increase the likelihood of fractures, especially in elderly populations already vulnerable due to aging and prior hormone therapies. Patients may also experience joint pain, fatigue, mood changes, and altered lipid profiles. Some men report hot flashes or gynecomastia, ironically due to estrogen imbalance, even as overall levels drop. Long-term suppression of estrogen in males may also impact cardiovascular health, libido, and emotional well-being. Careful pre-treatment counseling and regular follow-ups can help mitigate these side effects through early detection and supportive care such as bone-protecting agents, dietary strategies, or exercise programs.
Research Gaps and Future Directions
Despite the promising rationale for using aromatase inhibitors in prostate cancer, significant gaps remain in our clinical understanding. Most data to date are based on small cohorts or early-phase trials, with few long-term studies assessing outcomes like survival benefit or quality of life improvements. It is still unclear which patients are ideal candidates beyond ER-positive subtypes, and whether aromatase inhibition could be synergistic with newer modalities like immunotherapy or radioligand therapy. Furthermore, research is needed to explore the full spectrum of aromatase expression across different stages and grades of prostate cancer. The field would benefit greatly from large-scale randomized controlled trials and real-world registries that track patient outcomes, treatment resistance patterns, and biomarker evolution over time. Until such data is available, aromatase inhibition remains an experimental, albeit biologically plausible, strategy within a narrow patient segment.
Integrating Aromatase Inhibitors into Broader Prostate Cancer Therapy
Aromatase inhibitors should not be seen as a standalone option but rather as part of a broader therapeutic arsenal against prostate cancer. Their integration is most effective when aligned with a multi-modal approach that includes traditional androgen deprivation therapy, chemotherapy, radiation, and supportive care tailored to the patient’s clinical profile. In some cases, aromatase inhibitors may help prolong the period before more aggressive treatments are needed. In others, they might offer symptom control or serve as a salvage therapy when standard protocols fail. This integrative strategy parallels evolving treatments seen in other hormone-sensitive cancers. For instance, novel parallels have been drawn from estrogen-driven mechanisms observed in other contexts, such as breast-to-skin metastasis, where endocrine pathways can influence local tumor behavior (вставить ссылку: early breast cancer skin mets). Ongoing collaboration between endocrinologists, oncologists, and urologists is key to refining protocols and expanding safe use in clinical settings.
Frequently Asked Questions (FAQ)
What is androstenedione and why is it important in prostate cancer?
Androstenedione is a steroid hormone that serves as a precursor to both testosterone and estrogen. In prostate cancer, it becomes relevant because its conversion to estrogen can influence tumor growth in certain subtypes, making it a potential target for therapy.
How do aromatase inhibitors work in men with prostate cancer?
Aromatase inhibitors block the enzyme aromatase, which converts androstenedione and testosterone into estrogens. By reducing estrogen levels, these inhibitors may suppress tumor progression in estrogen-sensitive prostate cancers.
Are aromatase inhibitors FDA-approved for prostate cancer?
Currently, aromatase inhibitors are not FDA-approved specifically for prostate cancer treatment. Their use remains experimental and is generally guided by case studies or early clinical trials.
Can aromatase inhibitors be combined with other prostate cancer treatments?
Yes, they are often considered as adjunct therapies alongside androgen deprivation therapy (ADT), especially in hormone-sensitive or resistant disease cases.
Is there a way to test if a prostate tumor will respond to aromatase inhibitors?
Some tumors can be tested for estrogen receptor (ER) expression, but this is not standard practice in prostate cancer. Research is ongoing to identify reliable biomarkers that can predict response.
What are the main risks of using aromatase inhibitors in men?
Primary risks include decreased bone density, joint pain, cardiovascular effects, and hormonal imbalances that may impact mood and sexual function.
Can androstenedione levels be measured in routine blood tests?
Yes, specialized hormone panels can assess androstenedione levels, along with testosterone, estradiol, and other relevant markers to monitor hormonal status during treatment.
How does estrogen affect prostate cancer progression?
While testosterone is a primary driver of prostate cancer, estrogen also plays a role in certain tumor environments by promoting cell proliferation and influencing tumor microarchitecture.
Is there any evidence aromatase inhibitors can stop tumor growth?
Limited early-phase studies and case reports suggest that aromatase inhibitors may slow tumor progression in select patients, but definitive clinical evidence is still lacking.
Do aromatase inhibitors affect PSA levels?
Do aromatase inhibitors affect PSA levels?
Are there dietary ways to reduce aromatase activity naturally?
Some studies suggest that certain foods—such as cruciferous vegetables, mushrooms, and omega-3 fatty acids—may reduce aromatase activity, but these should not replace medical therapy.
How long can a patient stay on aromatase inhibitors?
Duration varies widely and depends on tolerance, response, and concurrent treatments. Long-term use requires monitoring for bone loss and other side effects.
Are there any alternatives to aromatase inhibitors for hormonal modulation?
Yes, alternatives include antiandrogens, GnRH agonists/antagonists, and other forms of endocrine therapy tailored to prostate cancer.
Why is aromatase inhibition not widely used in standard protocols?
Lack of large-scale clinical trial data and established biomarkers limits its incorporation into mainstream guidelines, though interest is growing in research settings.
Can estrogen suppression harm male patients long-term?
Prolonged estrogen suppression may lead to osteoporosis, cardiovascular risks, and mood disturbances, making risk-benefit assessments essential in treatment planning.









