Lassa Fever: A Persistent Threat in West Africa and Beyond
Introduction
What Is Lassa Fever, and Why Should We Care?
If you’ve never heard of Lassa fever, you’re not alone — and that’s part of the problem. For a disease that affects hundreds of thousands of people every year and can be fatal in severe cases, it tends to hover under the radar of global awareness. So let’s pull back the curtain.
Lassa fever is a viral hemorrhagic illness caused by the Lassa virus, a member of the Arenaviridae family — a group of viruses that are often zoonotic, meaning they jump from animals to humans. But unlike headline-grabbing viruses like Ebola, Lassa has a quieter, more insidious nature. It simmers in rural West African communities, flaring up into outbreaks that are devastating locally but rarely reach the global news cycle. Yet, its impact is profound — on public health systems, on communities, and on the global effort to manage emerging infectious diseases.
So, why care? Because diseases like Lassa fever remind us that epidemics aren’t just a matter of “if,” but “where” and “when.” And understanding the enemy is always the first step in reducing its power.
A Virus Rooted in a Region — But With Global Implications
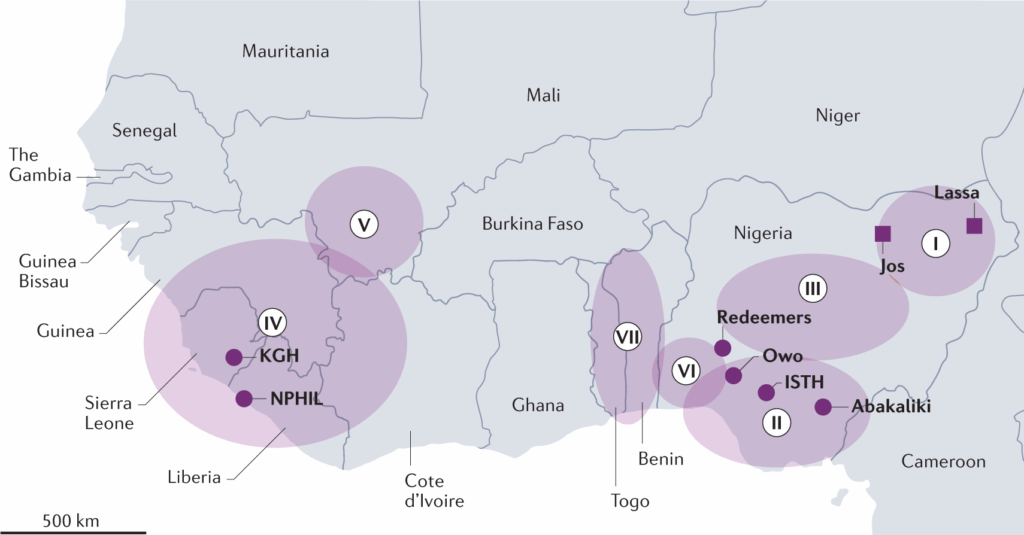
To grasp Lassa fever’s importance, we have to travel — at least in our minds — to West Africa, where this virus has established a persistent and often deadly relationship with human populations. The disease was first identified in 1969, in a town called Lassa in northeastern Nigeria, after two missionary nurses died of an unusual febrile illness. That initial outbreak was both a tragedy and a wake-up call: it exposed a virus that had likely been circulating for years, unrecognized and unrecorded.
Since then, Lassa fever has become recognized as endemic in several countries, including Nigeria, Sierra Leone, Liberia, and Guinea, with sporadic cases in neighboring nations and occasional travel-related cases abroad. In some parts of Nigeria, the disease is so common that hospitals operate with Lassa wards and protocols in place year-round — an eerie testament to how normalized this viral threat has become.
Despite this regional focus, the implications of Lassa fever are anything but local. In our increasingly interconnected world, any pathogen that spreads through contact and causes severe disease has the potential to spark wider concern. Moreover, its similarity to other hemorrhagic fevers, especially early in infection, makes it a diagnostic challenge — and a potential blind spot during outbreaks of febrile illness.
Why Lassa Fever Defies Easy Solutions
Here’s what makes Lassa fever particularly daunting: it doesn’t play by simple rules. About 80% of infections are mild or even asymptomatic, which means most people never know they have it — and yet, they can still spread the virus. The remaining 20% develop severe disease, with symptoms ranging from persistent fever and vomiting to internal bleeding and multi-organ failure.
Even more troubling is the unpredictability of who develops severe illness. It’s not always linked to obvious risk factors like age or preexisting conditions. And because early symptoms mimic more common diseases like malaria or typhoid, diagnosis is often delayed — sometimes fatally so.
We’re dealing with a virus that hides in plain sight, kills selectively but harshly, and has no widely available vaccine or targeted treatment. That makes it not only a clinical challenge but a public health puzzle.
A Place in the Viral Family Tree
Lassa virus belongs to the Old World arenaviruses, a subset of the Arenaviridae family. This viral family is divided into two groups: Old World and New World, based primarily on geography and genetics. The Old World group, which includes Lassa, is found mainly in Africa and causes diseases in humans, whereas the New World arenaviruses are more commonly associated with rodents in the Americas and include viruses like Junín and Machupo.
Arenaviruses are enveloped, single-stranded RNA viruses, and what makes them particularly interesting (and tricky) is their segmented genome — they have two RNA segments, one large (L) and one small (S), which code for different viral proteins. This kind of genomic architecture not only shapes how the virus replicates but also creates possibilities for mutation and reassortment, potentially influencing how the virus behaves over time. In short: Lassa is part of a family of shape-shifters.
The Bigger Picture: Lassa Fever as a Public Health Priority
The World Health Organization (WHO) has flagged Lassa fever as a priority pathogen for research and development, placing it alongside heavyweights like Ebola, Zika, and Nipah. That’s not just a bureaucratic label — it’s a call to action. These are diseases for which we lack effective countermeasures and which pose a significant risk of outbreaks. In other words, Lassa fever is on the radar, even if it’s not always in the headlines.
In many ways, Lassa fever resembles the kind of quiet, persistent threat that public health experts fear most — the ones that smolder in the background until, suddenly, they don’t. It’s the kind of scenario imagined in discussions of Disease X, where a pathogen’s potential lies not in dramatic headlines, but in overlooked patterns.
So in this series, we’re going to explore Lassa fever in all its complexity — not just as a virus, but as a phenomenon: a biological, ecological, social, and clinical challenge. We’ll dive into the molecular biology, trace its patterns across populations, unpack what it does to the body, and explore how medicine and science are fighting back.
Curious yet? You should be. Because Lassa fever isn’t just a regional illness — it’s a global lesson in vigilance, humility, and the importance of preparation in the age of emerging infectious diseases.
Virology and Pathogenesis
What Kind of Virus Is Lassa, Exactly?
To understand how Lassa fever works — how it infiltrates cells, spreads through the body, and wreaks havoc — we need to get to know its blueprint. Lassa virus is part of the Arenaviridae family, as we mentioned earlier, and it shares a lot of structural and functional traits with its arenavirus cousins. But don’t let the “family resemblance” fool you — Lassa has some specific tricks that make it particularly stealthy and dangerous.
Structurally, the virus is enveloped, meaning it has a soft outer layer derived from the host cell membrane. This envelope isn’t just decoration — it helps the virus sneak into human cells undetected. Beneath that envelope is the real action: the bisegmented RNA genome, which is divided into two strands of RNA known as the S segment (small) and the L segment (large). This segmented nature is important because it allows the virus to organize its genetic material efficiently and possibly adapt more quickly over time.
The S segment codes for two essential proteins: the nucleoprotein (NP) and the glycoprotein precursor (GPC). The L segment, on the other hand, encodes the RNA-dependent RNA polymerase (L) and a zinc-binding matrix protein (Z). Each of these components plays a role not just in viral replication but also in manipulating the host’s immune system. Yes, the virus isn’t just multiplying — it’s actively interfering with our ability to fight it.
A Trojan Horse Strategy: How Lassa Enters the Body
Let’s say someone gets exposed to the Lassa virus — through contact with contaminated food, rodent droppings, or infected human bodily fluids. What happens next?
The virus uses its surface glycoproteins to bind to a specific receptor on human cells known as alpha-dystroglycan (α-DG). This receptor is found in many tissues, including the epithelial cells lining the respiratory and gastrointestinal tracts, which is partly why the virus can cause such widespread symptoms. Once bound, the virus is taken into the cell through endocytosis, a kind of cellular “swallowing” process.
Now inside the cell, the virus begins uncoating and releasing its genetic material into the cytoplasm. From there, the viral RNA polymerase takes over, transcribing viral RNA and synthesizing new viral proteins. The infected cell essentially becomes a virus factory — churning out new virions that bud off and go on to infect neighboring cells. It’s efficient, it’s quiet, and it spreads fast.
Sabotaging the Alarm System: Lassa’s Immune Evasion Tactics
Here’s where things get really insidious. Most viruses trigger a strong immune response almost immediately — your body starts producing interferons, signaling proteins that alert other cells and rally your immune army. But Lassa virus has evolved to suppress that initial immune alarm.
Its nucleoprotein (NP) has an unusual ability: it can degrade viral double-stranded RNA, the molecular “red flag” that our cells use to detect intruders. By erasing these red flags, Lassa virus essentially flies under the radar. The immune system doesn’t realize there’s a problem until the virus has already established a stronghold. This delayed response is one reason the disease can progress rapidly and severely once symptoms begin.
Additionally, the Lassa virus may dampen T-cell responses, limiting the adaptive immune system’s ability to mount a focused, long-term attack. So not only does the virus start strong, it also weakens your best line of defense before your body can even organize it.
Why Some Get Sick — and Others Don’t
One of the great mysteries of Lassa fever is its variable presentation. Some people get infected and don’t even notice. Others develop severe hemorrhagic symptoms and organ failure. Why?
We don’t yet have a complete answer, but it likely involves a complex mix of host genetics, immune status, and viral load. Individuals with certain genetic polymorphisms in immune-related genes may mount more effective defenses, while others may be more susceptible to the virus’s immunosuppressive strategies. Also, the amount of virus someone is exposed to initially — and how quickly their immune system responds — probably plays a major role.
There’s also evidence that co-infections, malnutrition, and chronic health conditions can tip the balance toward more severe disease. In resource-limited settings, where health status is often already compromised, this makes Lassa fever particularly deadly.
What Happens in the Body: The Pathogenesis of Lassa Fever
Once the virus gains a foothold, it spreads primarily through the bloodstream and lymphatic system, targeting a wide range of tissues — including the liver, spleen, kidneys, and vascular endothelium. This widespread invasion explains the multi-system symptoms seen in severe Lassa cases.
The hallmark of Lassa pathogenesis is increased vascular permeability — in simple terms, the blood vessels start to leak. This leads to fluid shifts, hypovolemia (low blood volume), and shock, all of which are common in viral hemorrhagic fevers. Bleeding may occur, not necessarily due to blood vessel rupture, but because of platelet dysfunctionand impaired clotting.
In fatal cases, the virus overwhelms the immune system, causing a cytokine storm — a hyper-inflammatory state where the body’s own defense mechanisms begin damaging its tissues. In survivors, the immune system manages to contain the virus and initiate repair, but not without consequences. Some people develop long-term complications like hearing loss, which may be caused by immune-mediated damage to the inner ear structures.
A Molecular Arms Race Still in Progress
What’s fascinating — and slightly terrifying — is that Lassa virus doesn’t need to be especially flashy to be deadly. It doesn’t explode cells the way some viruses do. It doesn’t need airborne transmission or dramatic hemorrhaging in every case. Its strategy is one of stealth and suppression, slipping past the immune system, replicating broadly, and destabilizing core systems of the body like blood pressure and coagulation.
We’re still trying to fully map its interactions with the human immune system — and every insight we gain not only helps with treatment but offers a window into how other arenaviruses might behave.
Epidemiology
Where Does Lassa Fever Happen — and Why There?
Let’s start with a deceptively simple question: Where is Lassa fever found? The answer isn’t global — at least not yet — but it is geographically persistent, intensely regional, and deeply intertwined with environmental and socioeconomic conditions.
Lassa fever is endemic to parts of West Africa, meaning it’s not just an occasional visitor — it lives there. Countries like Nigeria, Sierra Leone, Liberia, and Guinea experience Lassa fever cases year-round, with periodic surges that sometimes take on epidemic proportions. In these regions, the virus isn’t rare. In fact, tens to hundreds of thousands of infections occur annually, though most are never diagnosed due to mild symptoms or limited access to testing.
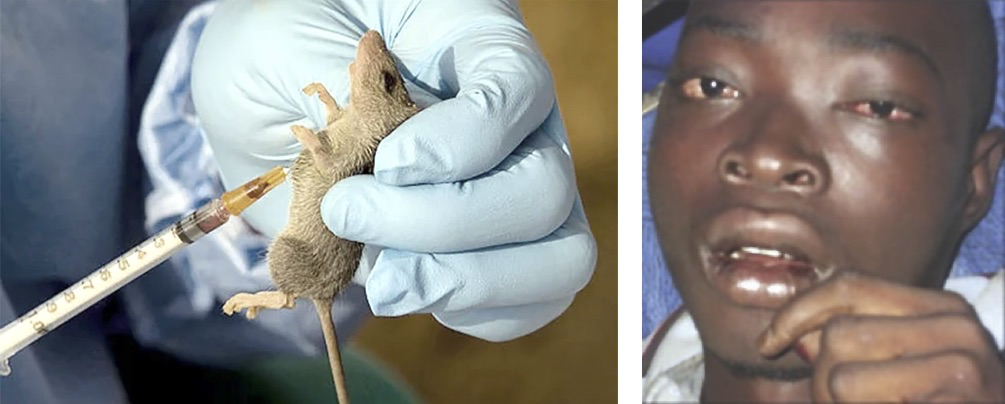
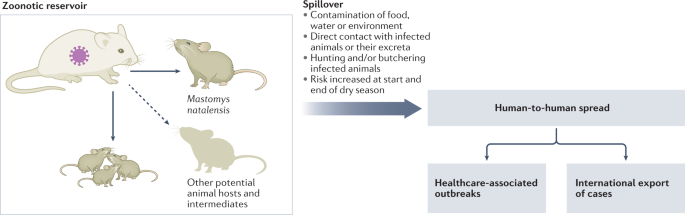
Why this region? It’s not a random coincidence. Lassa virus’s presence is closely linked to a very specific animal: the multimammate rat (Mastomys natalensis). This rodent species is widespread across sub-Saharan Africa, but its population density is particularly high in West Africa, and its habits — both ecological and behavioral — make it an ideal reservoir for the virus.
These rats aren’t shy. They live in and around human homes, rummage through food stores, and leave behind urine and feces — both of which can contain live virus. Humans who consume contaminated food, inhale aerosolized particles, or touch contaminated surfaces are at risk. It’s a setup that almost guarantees regular, low-level transmission — and occasionally, explosive outbreaks.
The Natural Reservoir: A Virus That Lives in Rats
Mastomys natalensis isn’t just the reservoir — it’s the ecological home of the Lassa virus. The rodents carry the virus chronically, without getting sick themselves, and they shed it throughout their lives. That’s important: Lassa virus doesn’t kill its host. From an evolutionary standpoint, this is genius. It ensures the virus’s survival and constant availability to jump into human populations.
Transmission from rodents to humans is primarily indirect — people rarely get bitten. Instead, the virus finds its way into human bodies via contaminated food, household surfaces, or inhalation of dust particles containing dried rodent excreta. In rural communities where storing food in rodent-proof containers is a luxury, and where grain may be dried on the ground or in open courtyards, these routes of exposure are difficult to eliminate.
And here’s something you might not expect: seasonality matters. Lassa fever incidence typically spikes during the dry season (December to April). During this time, agricultural practices shift, people store more food indoors, and rodent-human contact increases. But why exactly this period is worse remains an area of ongoing study — ecology, climate, and human behavior are all suspects.
Person-to-Person Transmission: Yes, It Happens — But Not Like the Flu
One of the questions that often comes up: Can you catch Lassa fever from other people? The answer is yes — but not easily, and not always.
Lassa virus can be transmitted from human to human, especially in healthcare settings, where exposure to infectious blood, vomit, urine, feces, or other bodily fluids is more likely. Caregivers, family members, and health workers without proper protective equipment are particularly vulnerable. In fact, nosocomial (hospital-acquired) outbreaks are a recognized and dangerous feature of Lassa fever, especially where infection control practices are limited or overwhelmed.
But unlike influenza or COVID-19, Lassa fever doesn’t typically spread through casual respiratory droplets. That’s a key reason why, despite its lethality, it hasn’t gone global. It’s not airborne in the conventional sense — it requires closer contact, bodily fluid exposure, or contaminated surfaces. That gives public health teams a fighting chance to contain outbreaks when they’re detected early.
Still, the risk of superspreading events in healthcare facilities is real. In some documented outbreaks, a single undiagnosed patient has led to dozens of secondary infections, especially when invasive procedures (like intubation or surgery) are performed without adequate protection.
Who Gets Lassa Fever — and Who’s Most at Risk?
The virus doesn’t discriminate by age or gender, but certain groups are more vulnerable simply due to exposure risk. People living in rural areas, especially those involved in agriculture, food storage, or grain processing, face a higher likelihood of encountering infected rodents or their droppings. Children, too, are often exposed in households where rodent control is minimal and food safety is hard to enforce.
And let’s talk about healthcare workers. In endemic areas, doctors, nurses, lab technicians, and even janitorial staff are frequently at the frontlines of Lassa exposure. In poorly equipped hospitals, where gloves, masks, or even running water may be scarce, the virus exploits every vulnerability in the system.
There’s also concern for pregnant women — not only are they more likely to develop severe disease, but Lassa virus can cross the placenta, often resulting in miscarriage or fetal death. Maternal mortality from Lassa fever is also heartbreakingly high, making it a serious concern in maternal health in endemic regions.
Silent Carriers: The Invisible Part of the Epidemic
One of the most intriguing — and complicating — features of Lassa epidemiology is how many people carry the virus without showing symptoms. It’s estimated that up to 80% of infections are mild or asymptomatic, meaning the person may never know they were infected. That sounds like a good thing, and for the individual, it usually is. But from a public health standpoint, it creates a major problem.
These silent carriers can still shed virus, particularly early in infection, contributing to transmission chains without triggering alarms. And because many of the early symptoms are nonspecific — think fever, malaise, sore throat — cases are frequently misdiagnosed as malaria, typhoid fever, or even the flu.
This diagnostic ambiguity delays treatment, complicates contact tracing, and increases the risk of outbreaks growing undetected. It also means that many Lassa-related deaths may go unrecorded or misattributed to other causes, leading to an underestimation of the disease’s true burden.
Patterns That Are Shifting: Urban Spread and Climate Influence
Traditionally, Lassa fever has been seen as a rural disease. But this is beginning to change. As urbanization continues across West Africa, rats — ever-adaptable — are following people into cities. With increasing population density and often inadequate sanitation, urban outbreaks are becoming a real possibility.
And then there’s climate change, which is subtly altering the landscape of rodent populations and viral transmission. Changing rainfall patterns, deforestation, and shifting agricultural zones may all influence how and where Mastomyspopulations thrive — and, by extension, where Lassa virus can spread. These aren’t just abstract concerns; they represent real shifts that could expand the virus’s geographic reach or alter its seasonal patterns.
The challenge with Lassa — as with so many underdiagnosed conditions — is that it doesn’t always look like what it is. Much like how researchers are learning to extract hidden signals from routine CBC tests to detect cancer early, there’s a growing push to develop tools that can catch Lassa before it announces itself with full-blown symptoms.
Clinical Manifestations
What Does Lassa Fever Feel Like? Spoiler: It’s Not Always Dramatic
You might expect a hemorrhagic virus to announce itself with drama — fevers, rashes, visible bleeding, the works. And in some cases, Lassa fever can look like that. But more often? It starts quietly. Subtly. A bit of fatigue here, a sore throat there. Maybe a mild fever that’s all too easy to shrug off as “just malaria again.”
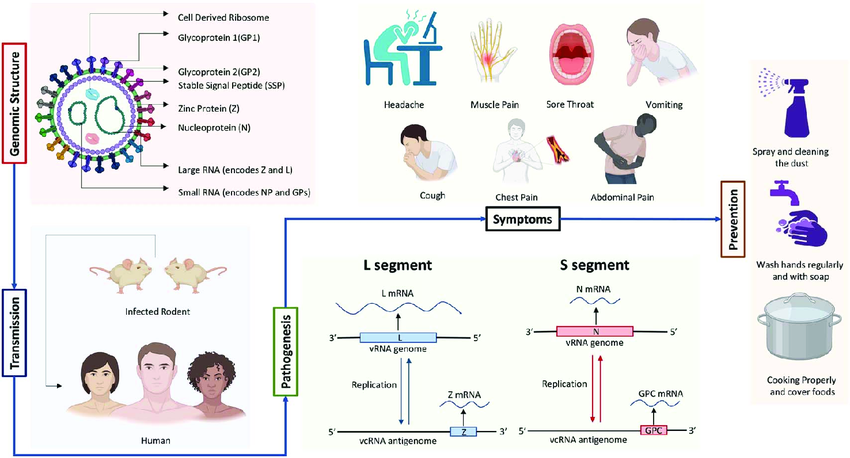
This is one of the defining frustrations of Lassa fever. In its early stages, it mimics dozens of more common illnesses, particularly in West Africa where febrile diseases are everywhere. Malaria, typhoid, influenza, even dengue — they can all look and feel like the first few days of Lassa. That means patients often delay seeking care, and clinicians may treat the wrong thing first. Time is lost, and in some cases, so is the chance of recovery.
So what are we actually dealing with?
The First Wave: Early Symptoms That Fool Everyone
The incubation period for Lassa virus is typically 6 to 21 days — so someone may go weeks between exposure and illness. Once symptoms do appear, they tend to start gradually, not explosively.
Common early symptoms include:
- Fever — often persistent, sometimes with chills.
- Malaise and general weakness — that frustrating “I just don’t feel right” sensation.
- Headache and sore throat — especially painful sore throat, sometimes with ulcers.
- Muscle aches and back pain — nonspecific but debilitating.
There may also be abdominal pain, nausea, or diarrhea, which again overlap heavily with gastrointestinal infections. This phase can last for several days and, in many people, it begins to resolve on its own. These individuals will never be diagnosed unless blood testing is done — and often, it isn’t.
But for others, the virus continues to spread — and the disease evolves into something far more dangerous.
The Second Wave: Severe and Systemic Disease
Roughly 20% of Lassa fever infections progress to severe disease, and when they do, things can deteriorate rapidly. This phase is where we see the hallmarks of viral hemorrhagic fever, although bleeding is not universal.
Severe symptoms can include:
Hemorrhagic manifestations
These might involve bleeding from the gums, nose, gastrointestinal tract, or even the eyes. However, bleeding is not as common or as profuse as in diseases like Ebola — and its absence does not rule out Lassa. In fact, many severe cases never involve visible bleeding at all.
Respiratory involvement
Patients may develop cough, chest pain, or difficulty breathing, particularly as fluid accumulates or as secondary infections take hold. Sometimes the lungs themselves become inflamed, or fluid leaks into surrounding tissues due to damaged blood vessels.
Neurological signs
In more advanced cases, Lassa virus can affect the central nervous system. Confusion, seizures, tremors, and even coma have been observed — though these are late signs and usually portend a very poor prognosis.
Hepatic and renal dysfunction
Liver and kidney failure are major contributors to fatal outcomes. Elevated liver enzymes, jaundice, and reduced urine output are all red flags. These organs are especially vulnerable to the virus’s tendency to damage vascular structures and interrupt oxygen supply.
Shock
Ultimately, many patients who die from Lassa fever do so from hypovolemic shock — their blood pressure plummets due to fluid loss and vascular leakage. It’s not always bleeding that causes it; often, it’s fluid simply leaking into tissues where it doesn’t belong.
What Makes It Fatal? Complications and Red Flags
The case fatality rate of Lassa fever varies widely. For the general population, it’s around 1%, which might not sound high — but that’s among all infections, including the mild ones. Among hospitalized patients, the mortality rate jumps to 15%–20%, and in severe cases, particularly those with organ failure or neurological involvement, it can reach 50% or higher.
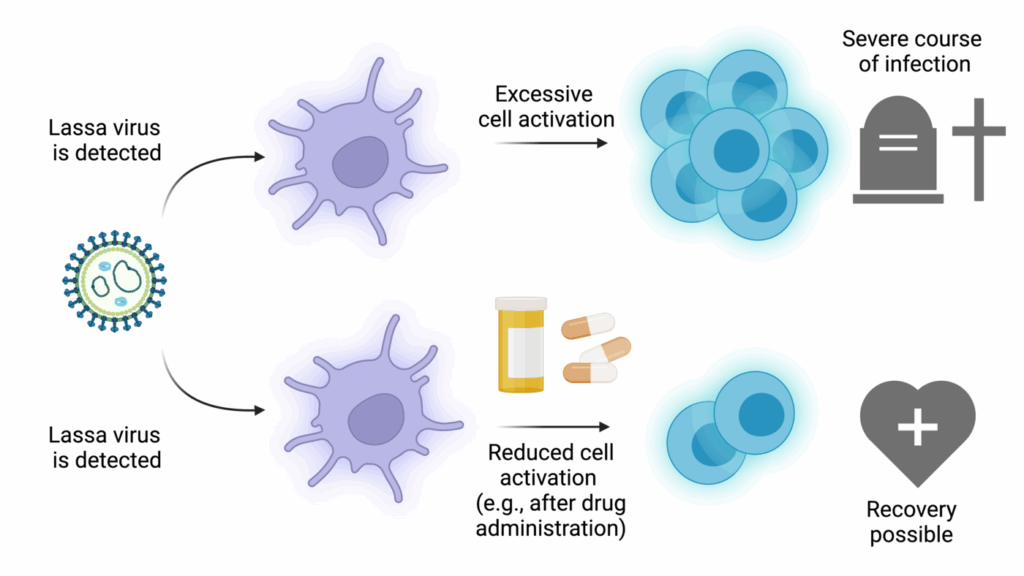
Pregnant women, especially in the third trimester, are particularly at risk. The virus can cause spontaneous abortion, and maternal mortality can be shockingly high — in some studies, over 80% of pregnant women with Lassa fever in the third trimester died.
Another frequent and cruel complication is sensorineural hearing loss. It may develop in the recovery phase — or even during mild infections — and in some cases, it becomes permanent. The mechanism isn’t fully understood, but it’s thought to be due to immune-mediated damage rather than direct viral attack on the auditory system. What’s worse: this hearing loss can occur in patients who otherwise recover completely.
Other long-term complications may include chronic fatigue, memory issues, and muscle weakness, but more research is needed to map these fully.
Recovery: A Slow, Uneven Road
For survivors, recovery isn’t always quick or complete. Fatigue can linger for weeks or even months. If hearing loss occurs, it may not improve. Psychological effects, especially in those who were severely ill or lost family members, can be profound. And in resource-limited settings, access to rehabilitation, audiology, or mental health care is often nonexistent.
There’s also the question of viral persistence. In some survivors, the virus may linger in immune-privileged sites — areas of the body like the eyes, testes, or central nervous system, where immune surveillance is weaker. This raises important questions about whether late complications, relapses, or even sexual transmission could occur — and though not as well documented as with Ebola, it remains an active area of research.
Diagnosis
How Do You Diagnose a Virus That Looks Like Everything Else?
This is one of the central challenges of Lassa fever — spotting it early, and spotting it accurately. Because here’s the frustrating truth: in the early stages, Lassa fever looks like everything and nothing all at once.
Fever, headache, sore throat, maybe some vomiting. Sounds familiar, right? In West Africa — the region where Lassa is endemic — malaria is everywhere, and so is typhoid. Even the common cold can start this way. So when someone walks into a rural clinic feeling lousy and burning up, Lassa fever is rarely the first diagnosis that comes to mind — and that’s a problem.
Because Lassa’s early clinical ambiguity is exactly what allows it to spread undetected, especially in community or hospital settings. Every hour without a correct diagnosis is an hour the virus can move on to someone else.
So how do we diagnose it?
Laboratory Diagnosis: The Only Reliable Route
Let’s make this clear: there is no way to definitively diagnose Lassa fever based on symptoms alone. Clinical suspicion is important — sometimes life-saving — but confirmation requires laboratory testing. And in many endemic areas, access to these tests is limited, delayed, or simply nonexistent.
So what’s in the diagnostic toolkit?
RT-PCR: The Gold Standard (When Available)
Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) is the most reliable method for detecting Lassa virus in blood samples during the acute phase of illness. It works by detecting viral RNA — and it’s highly sensitive when done correctly.
But here’s the catch: PCR requires well-equipped labs, trained personnel, and a secure chain of sample transport — all things that aren’t guaranteed in rural West African settings. Delays in getting samples to central labs can result in diagnosis coming too late to help the patient — or prevent further spread.
In Nigeria, for example, there are designated Lassa fever testing centers, but not every hospital has one. Samples often have to be shipped to a few centralized labs, which adds precious time. And if a healthcare worker isn’t already suspicious of Lassa, the test may not even be ordered.
Serological Testing: Looking for Antibodies
In cases where PCR isn’t possible, or when the patient presents later in the illness, serological tests may be used to detect IgM or IgG antibodies against the Lassa virus. These tests can indicate whether a person has recently been infected or had past exposure.
The problem? They aren’t always helpful in the early days of infection, because antibodies take time to appear — and by then, the most critical window for intervention may have passed.
Antigen Detection Tests: Rapid, but Still Under Development
Some rapid diagnostic tests (RDTs) that detect Lassa virus antigens — the actual proteins from the virus — are being developed and tested. These would be game-changers if widely deployed: imagine a point-of-care strip test, like a pregnancy test or malaria RDT, but for Lassa.
Unfortunately, most are still in the experimental phase or have limited field use. Getting them approved, manufactured, and distributed across endemic areas remains a challenge, although progress is being made — and we’ll touch on this again in Part Eight.
The Danger of Misdiagnosis: Not Just a Missed Case
Misdiagnosing Lassa fever isn’t just a clinical error — it can have public health consequences. If a case is mistaken for malaria or typhoid, no precautions are taken to prevent transmission. The patient might be admitted to a general ward, cared for by unprotected staff, or undergo procedures that aerosolize infectious fluids.
This is exactly how nosocomial (hospital-acquired) outbreaks have begun in the past. And it’s also why healthcare workers in endemic regions must maintain a high index of suspicion, especially when a patient has:
- Fever unresponsive to antimalarials or antibiotics
- A history of rodent exposure or residence in endemic areas
- Unusual bleeding or swelling
- Close contact with another sick person showing similar symptoms
These red flags can save lives — but only if recognized.
Diagnostic Gaps and the Need for Field-Ready Solutions
The reality is sobering. Timely diagnosis of Lassa fever still depends heavily on geography and luck. If you’re a patient in a capital city, near a referral hospital with lab capacity, you might be diagnosed within a day or two. But if you live in a rural village — even just 100 kilometers away — your chances of getting tested in time drop significantly.
This diagnostic inequity mirrors broader health system gaps. Better tests aren’t enough; we also need better distribution, better training, and stronger referral networks. In other words, diagnosis doesn’t begin in the lab — it begins in the clinic, with suspicion and timely action.
We also need to empower community health workers, many of whom are the first point of contact in remote areas. If they’re trained to recognize warning signs and know when to refer patients, the diagnostic chain begins much earlier — and more lives can be saved.
Beyond Diagnosis: Surveillance and Preparedness
It’s easy to think of diagnosis as a one-time event — confirm the case, treat the patient. But in public health terms, diagnosing a case of Lassa fever is like discovering a spark in dry grass. That one case could represent dozens of missed infections, and more could be smoldering quietly nearby.
That’s why confirmed diagnoses also trigger epidemiological surveillance: contact tracing, alerting nearby hospitals, checking for clusters. Good diagnostics aren’t just clinical tools — they’re epidemic intelligence systems. And in regions where Lassa is endemic, that intelligence can mean the difference between a contained outbreak and a regional crisis.
Treatment and Management
So You Have Lassa Fever — Now What?
Let’s say you’ve made it past the diagnostic minefield. Lassa fever is confirmed. What comes next?
The answer, frustratingly, is that we’re still mostly in supportive care territory. Unlike diseases with well-established antiviral regimens (like HIV or influenza), Lassa fever doesn’t yet have a universally effective treatment protocol. What we do have is a combination of timing, clinical intuition, aggressive supportive therapy, and — in select cases — a drug called ribavirin.
But before we get to the pharmacology, let’s talk about what “supportive care” really means in the context of a virus that can cause multi-organ failure and hemorrhagic shock.
Supportive Care: The Unsung Hero
It might sound unimpressive, even generic: “supportive care.” But in the hands of a skilled clinical team, supportive care can save lives — especially when given early.
Fluid management
Lassa fever often leads to hypovolemia — low blood volume — due to vomiting, diarrhea, vascular leakage, and sometimes bleeding. Maintaining fluid balance is critical, but tricky. Give too little, and the patient goes into shock. Give too much, and you risk pulmonary edema (fluid in the lungs), especially in patients with compromised kidney function.
Experienced clinicians often walk this tightrope using intravenous fluids, electrolyte monitoring, and careful clinical judgment — a skill that’s harder to apply in low-resource settings without laboratory support.
Oxygen support
In severe cases, oxygen therapy may be needed. When lungs begin to fill with fluid or secondary infections set in, hypoxia can become a major issue. Ventilators, of course, are rare in rural West African hospitals — so this means oxygen cylinders or concentrators, which come with their own logistical challenges.
Treating secondary infections
Because Lassa virus weakens the immune response, opportunistic bacterial infections are common. Patients often require broad-spectrum antibiotics alongside other therapies. Again, access and timing are everything.
Monitoring for organ failure
Kidney and liver function can deteriorate rapidly. In some cases, dialysis might be indicated — but again, that’s a rare option outside of specialized centers. Where lab testing is available, urea, creatinine, ALT/AST levels, and platelet counts are monitored closely.
Supportive care may sound basic on paper, but in practice, it requires a highly trained team, a well-equipped facility, and constant vigilance — which is why the Lassa fatality rate varies so widely depending on where a patient is treated.
Ribavirin: A Drug With Potential — and Problems
For decades, ribavirin, a broad-spectrum antiviral, has been the only drug used to treat Lassa fever. And to be clear, it can be life-saving — but only when given early.
Ribavirin works by interfering with viral RNA synthesis, effectively slowing or halting viral replication. However, it has a few major caveats:
- Timing is critical. The drug is most effective when administered within the first 6 days of illness — a timeframe that’s often missed due to diagnostic delays.
- Dosing is aggressive. Ribavirin treatment for Lassa isn’t a single pill. It involves high-dose intravenous administration, often over 10 days, and the regimen can be difficult to manage.
- Side effects exist. Ribavirin isn’t without risks. It can cause anemia, liver toxicity, and teratogenic effects (birth defects if used during pregnancy), which means it’s contraindicated for many pregnant women — ironically, the group at highest risk of death from Lassa.
- Availability varies. In endemic regions, ribavirin is often in short supply, and costs can be prohibitive. Many facilities simply can’t afford to keep it stocked year-round.
So while ribavirin remains the backbone of antiviral treatment, its limitations highlight the urgent need for better therapeutics.
Investigational Therapies: What’s in the Pipeline?
Fortunately, the tide is beginning to shift. In the past few years, a number of experimental drugs and monoclonal antibody therapies have entered preclinical and early clinical trials for Lassa fever. Here are a few promising directions:
Monoclonal antibodies
Inspired by the success of antibody therapies in treating Ebola, researchers are now developing neutralizing monoclonal antibodies targeting Lassa virus glycoproteins. These could be used for post-exposure prophylaxis (PEP) or early treatment.
Initial results in animal models have been encouraging — showing both therapeutic and preventive potential — but human trials are still in progress, and regulatory hurdles remain.
Broad-spectrum antivirals
Compounds like favipiravir (used for influenza and investigated for COVID-19) have shown some efficacy against arenaviruses in lab settings. These drugs are appealing because they may work across multiple viruses — but dosing, safety, and real-world effectiveness are still being studied.
Immunomodulators
Some approaches aim not at the virus itself, but at the host immune response — trying to prevent the cytokine storms and immune dysregulation that lead to severe disease. These therapies are still highly experimental but reflect a more nuanced understanding of Lassa pathogenesis.
Vaccines: Are We Close?
The idea of a Lassa fever vaccine is not new — but for a long time, progress was slow. In recent years, however, momentum has picked up, thanks to coordinated efforts by groups like CEPI (Coalition for Epidemic Preparedness Innovations), WHO, and academic partners.
Several candidate vaccines are now in preclinical or early-phase human trials, including:
- DNA-based vaccines, which use segments of Lassa virus genetic material to train the immune system.
- Viral vector vaccines, similar to the ones used for Ebola and COVID-19, which deliver Lassa antigens via a harmless carrier virus.
- mRNA vaccines, still in the experimental stage, but promising given the recent advances in mRNA vaccine technology globally.
The biggest hurdles aren’t just scientific. They’re logistical and financial: funding for trials, ensuring ethical standards, and preparing for large-scale deployment once a vaccine is ready. But optimism is growing, especially as global health organizations increasingly view Lassa as a pathogen of epidemic potential.
Clinical Infrastructure and Training: The Other Half of the Equation
Even with drugs and vaccines, you can’t treat what you can’t find. That’s why strengthening clinical capacity is just as important as biomedical breakthroughs.
Many endemic countries are developing Lassa-specific treatment centers, complete with trained staff, isolation wards, PPE stocks, and emergency response protocols. These hubs are not only essential for care — they’re also training grounds for future clinicians and testing grounds for new treatments.
But still, the urban-rural divide looms large. Getting a patient from a village to a treatment center in time can mean the difference between life and death — and too often, the roads, ambulances, or funds simply aren’t there.
Prevention and Control
Can We Really Prevent Lassa Fever? Short Answer: Yes… But It’s Complicated
You might be wondering — if we know Lassa fever is caused by a virus that lives in rats, and we know how it spreads, then shouldn’t prevention be relatively straightforward?
It seems like it should be. In theory, preventing Lassa fever is a matter of avoiding exposure to the virus, primarily by limiting contact with the rodent reservoir and containing person-to-person transmission when outbreaks occur. But as is so often the case in global health, what’s simple in theory becomes layered and difficult in practice — especially in regions where poverty, infrastructure gaps, and cultural practices complicate everything.
Still, prevention isn’t a lost cause. In fact, many of the most effective measures are low-tech, community-driven, and entirely achievable — if given the proper support and resources.
Let’s walk through what that looks like.
At the Root: Controlling the Rodent Reservoir
It’s impossible to talk about Lassa prevention without addressing Mastomys natalensis, the multimammate rat. These rodents are not just the main reservoir — they’re practically neighbors in many parts of West Africa. They live in and around homes, food stores, markets, and fields. They’re nocturnal, prolific breeders, and they’re not particularly fearful of humans.
So how do you keep people and rats apart?
Household sanitation and food security
This is the frontline of prevention. Simple measures — like storing grain in sealed containers, keeping cooking areas clean, and disposing of food waste properly — can significantly reduce the risk of rodent attraction. But these aren’t always easy changes to make.
Many homes in Lassa-endemic areas are constructed from mud or thatch, with open eaves, gaps in walls, and dirt floors— perfect environments for rodents to enter and nest. Upgrading housing structures is expensive. And even when rodent-proof materials are available, cultural norms and resource constraints may slow adoption.
Rodent control efforts
Trapping and killing rats is one approach, but mass poisoning or eradication campaigns can backfire. They may cause rodents to migrate unpredictably or lead to contamination of human water supplies. More sustainable approaches involve habitat control — sealing entry points in homes, elevating food storage, and encouraging natural predators like cats.
Community education plays a huge role here. When people understand that rodent contact = virus exposure, they’re more likely to prioritize these preventive steps — even if they don’t always have the resources to implement them fully.
Preventing Human-to-Human Transmission: The Hospital as a Hotspot
Let’s pivot to another crucial setting: the healthcare facility.
Lassa virus, as we know, can spread from person to person through blood, bodily fluids, and contaminated surfaces. This makes hospitals and clinics high-risk environments — especially in the early stages of an outbreak when the diagnosis is unclear, and protective measures may not yet be in place.
Infection prevention and control (IPC)
Proper IPC practices — gloves, gowns, masks, hand hygiene, patient isolation — can dramatically reduce nosocomial transmission. But this is where theory again meets reality.
In many rural hospitals across West Africa, basic supplies run low. Gloves may be reused. Soap and water may be scarce. Dedicated isolation rooms might not exist. And when healthcare workers fall ill — as they have in multiple Lassa outbreaks — the human cost is devastating, both for the individuals and the healthcare system.
That’s why training is as essential as equipment. Knowing how and when to suspect Lassa, how to safely triage patients, and how to protect oneself during high-risk procedures is a life-saving skill set.
Contact tracing and case isolation
Once a case is confirmed, rapid isolation and contact tracing are critical. The goal is to identify and monitor everyone who’s been in close contact with the patient — family members, hospital staff, neighbors — and watch for symptoms over a 21-day window.
This process requires trust. In some communities, Lassa isolation units are feared — often seen as places where people go in and don’t come out. Effective outreach and communication are essential to dispel fear and encourage cooperation. It’s not just science; it’s relationship-building.
Community Education: The First and Best Line of Defense
So many Lassa prevention strategies hinge on human behavior. And that means education is power — not just clinical knowledge, but culturally grounded, accessible public health messaging.
What does that look like in practice?
- Explaining how rodent urine or feces can contaminate food.
- Teaching proper hand hygiene and waste disposal.
- Addressing myths — for instance, that Lassa is a curse or punishment, or that it can’t be survived.
- Engaging local leaders, traditional healers, and religious figures to spread accurate information in trusted spaces.
Health education campaigns — delivered via radio, community health workers, or local gatherings — can make a tangible difference in how communities respond to Lassa outbreaks. And in areas where fear of hospitals or stigma prevents early presentation, education can open the door to earlier treatment and better outcomes.
Border Control and International Coordination
Though Lassa fever is still regionally contained, cases have been exported before — typically by travelers who become symptomatic after leaving an endemic country. This has triggered international alerts, airport screenings, and in rare cases, quarantine procedures abroad.
That’s why international coordination matters. Health authorities in Lassa-endemic countries work closely with WHO and neighboring nations to report cases, track outbreaks, and prevent cross-border transmission.
But even more critically, regional cooperation helps in the development of shared laboratory networks, referral systems, and emergency response protocols — so that a case in rural Nigeria doesn’t become a crisis in coastal Guinea or beyond.
The Long Game: Building Systems That Outlast the Outbreak
At the heart of Lassa prevention is a bigger truth: the same systems that prevent Lassa fever are the ones that make health systems resilient overall.
When we invest in:
- Reliable primary care facilities,
- Health worker training,
- Lab infrastructure,
- Community outreach,
- Sanitation and housing improvements,
—we’re not just preventing Lassa. We’re building the foundation to detect and respond to other emerging infectious diseases, too. This is why organizations like WHO and CEPI consider Lassa fever a model case for how to strengthen epidemic preparedness from the ground up.
Recent Developments (2025–2026)
What’s New in the World of Lassa Fever?
For decades, Lassa fever has been a bit of a medical underdog — a dangerous, persistent disease, but one that never quite captured the global spotlight. That’s changed in recent years. Thanks to increased attention from international health organizations, advancements in science, and several high-profile outbreaks, Lassa fever is now firmly on the global health radar.
And that attention is starting to pay off.
From breakthroughs in rapid diagnostics to promising new treatments and vaccine candidates, the landscape of Lassa research and response is shifting. Let’s explore what’s happened in the past two years — not just what’s new, but what’s meaningful.
Notable Outbreaks: A Pattern of Escalation — and Awareness
Nigeria: A Consistent Epicenter
Nigeria remains the country most heavily affected by Lassa fever, and the 2025 dry season brought another surge. According to the Nigeria Centre for Disease Control (NCDC), case numbers were slightly lower than the 2023–2024 peak, thanks in part to earlier detection and more consistent use of infection prevention protocols in hospitals. However, several states — including Edo, Ondo, and Bauchi — still reported record-high case fatality rates in rural clinics with delayed access to care.
The outbreak also emphasized an ongoing concern: the rural-urban gap in diagnostics. In Lagos, cases were quickly tested and managed. In smaller towns, by contrast, it often took days to get a diagnosis — days that made the difference between life and death.
Cross-Border Coordination
In 2025, Benin and Togo each reported clusters of suspected Lassa fever cases near the border with Nigeria — and for the first time, joint response teams were deployed between countries, coordinated by the West African Health Organization (WAHO). This marked a turning point in regional surveillance cooperation, reflecting a growing understanding that Lassa doesn’t respect borders, and neither should our response.
Diagnostics: Speed Is Finally Catching Up With Urgency
One of the most exciting advances of 2025–2026 has been the rollout of rapid diagnostic tests (RDTs) for Lassa fever — field-deployable, point-of-care tools that can deliver results in under 30 minutes.
These RDTs detect Lassa virus antigens in a patient’s blood, and several have now passed WHO emergency use listingfor deployment in outbreak settings. They’re not as sensitive as lab-based PCR, but they’re accurate enough to guide early triage and isolation, especially in under-resourced clinics where speed matters most.
And here’s what’s powerful: these tests are simple to use, require no electricity, and can be stored without refrigeration. That makes them ideal for community health workers, who are often the first line of contact in rural areas.
While these RDTs won’t replace PCR entirely, they represent a paradigm shift — bringing Lassa diagnosis closer to where patients actually live and seek care.
Treatments: The Era of Targeted Therapy May Be Near
2025 saw the conclusion of the first Phase II clinical trial of a monoclonal antibody therapy for Lassa fever. The trial, conducted jointly by NIH and West African research partners, tested a cocktail of neutralizing antibodies designed to block viral entry into cells.
Preliminary results showed:
- Reduced viral load within 48 hours
- Improved survival rates when administered early
- Minimal side effects, even in critically ill patients
This therapy, now advancing into Phase III trials, may eventually be stockpiled alongside ribavirin as a first-response treatment for confirmed cases and post-exposure scenarios — especially for healthcare workers or pregnant women who are at highest risk.
In parallel, antiviral research has also accelerated. A candidate called LVR-405, a novel arenavirus inhibitor, entered animal testing in late 2025 with promising efficacy against multiple arenaviruses, including Lassa. It’s still early days, but the trend is clear: we’re no longer stuck with a single drug.
Vaccine Progress: A Breakthrough on the Horizon?
The biggest headline from 2025 so far? The launch of a large-scale human trial for a Lassa fever vaccine in Nigeria and Sierra Leone.
Developed by a coalition led by CEPI and the Jenner Institute at Oxford, this viral vector vaccine uses a harmless chimpanzee adenovirus to deliver Lassa virus glycoprotein antigens. In preclinical studies, it generated robust immune responses in non-human primates and protected against lethal challenge.
The trial — known as STRIVE-LF — will enroll over 3,000 adults, including healthcare workers, market vendors, and agricultural workers. Participants will be monitored for two years, with endpoints focusing on infection rates, immune markers, and safety.
If successful, this could lead to the world’s first licensed Lassa fever vaccine, and pave the way for preventive strategies in outbreak-prone zones.
Public Health Systems: Signs of Strengthening
While science is surging ahead, there’s also good news on the systems front. In 2025–2026:
- Nigeria expanded its Lassa diagnostic network, adding five new regional testing labs equipped with PCR capacity and cold-chain systems.
- Community-based surveillance programs were launched in 50 rural districts across Nigeria and Sierra Leone, training over 1,200 local health workers in early detection and patient referral.
- Mobile alert systems were piloted, using SMS and voice messages to disseminate outbreak warnings and hygiene tips in local languages — reaching tens of thousands in real-time.
These may sound like small steps, but they reflect a shift from reactive crisis management to proactive epidemic readiness — and that’s exactly what’s needed.
Global Interest, Local Voices
Finally, it’s worth noting how much local leadership has shaped the recent response. More Lassa researchers, clinicians, and public health leaders are now based in West Africa, not just parachuted in from international institutions.
This matters. Local scientists are asking sharper questions, rooted in community realities. Local health ministries are pushing for sustainable infrastructure, not just emergency surge funding. And local patients — survivors, families, and volunteers — are increasingly visible in advocacy and education efforts.
Much like the evolving picture of avian influenza H5N1, Lassa fever reminds us that even long-known viruses can surprise us — adapting, reemerging, or expanding their reach when conditions shift just enough.
Lassa fever may have been neglected once, but those days are numbered. A new generation of response is emerging — one that blends high-tech innovation with grassroots power.
Frequently Asked Questions (FAQ)
1. Is Lassa fever contagious?
Yes, but not in the way most people think. Lassa fever doesn’t spread easily through casual contact or the air. Transmission requires direct contact with the bodily fluids (blood, urine, feces, vomit) of an infected person or exposure to contaminated surfaces. Most human-to-human transmission occurs in hospital settings or households during close, unprotected care.
2. Can Lassa fever be cured?
There’s no guaranteed cure, but early treatment significantly improves survival. The antiviral ribavirin can reduce disease severity if given within the first week of illness. In 2025–2026, monoclonal antibody therapies and new antivirals entered clinical trials, showing promise. Still, the cornerstone of management is supportive care — fluids, oxygen, and infection control.
3. Is there a vaccine for Lassa fever?
Not yet — but we’re closer than ever. As of 2025, several vaccines are in advanced clinical trials, including a viral vector vaccine developed by the Jenner Institute. Large-scale trials are underway in West Africa, and if results are favorable, a licensed vaccine could be available in the next few years.
4. How does someone catch Lassa fever in the first place?
The primary source is Mastomys natalensis, the multimammate rat that lives in and around homes in West Africa. People usually get infected by eating food contaminated with rat urine or droppings, or by inhaling airborne particles stirred up from nesting material or rodent waste.
5. What are the early symptoms of Lassa fever?
Lassa fever often starts mildly, with symptoms like fever, sore throat, muscle aches, nausea, and general weakness. This makes it easy to confuse with malaria or typhoid. In severe cases, it can progress to bleeding, shock, respiratory distress, and organ failure.
6. Can you get Lassa fever more than once?
It’s rare. Infection typically results in long-lasting immunity, though the exact duration and strength of that immunity is still being studied. Reinfection is theoretically possible, but not commonly reported.
7. Why is Lassa fever mostly found in West Africa?
Because that’s where Mastomys natalensis thrives, and where living conditions often bring people into close contact with rodents. That ecological relationship, combined with limited access to healthcare and diagnostics, has allowed the virus to establish itself as an endemic disease in the region.
8. What’s the long-term outlook for survivors?
Most survivors recover fully, but long-term complications can occur. Hearing loss is the most common — sometimes partial, sometimes permanent — and may affect up to a third of survivors. Fatigue, weakness, and psychological effects can also linger for weeks or months.
9. How can people protect themselves from Lassa fever?
At the individual level, prevention includes storing food securely, keeping homes clean and rodent-proof, and using protective equipment when caring for sick individuals. On a larger scale, public health education, rodent control, and improved clinical infrastructure are key to reducing spread.
Conclusion
What Lassa Fever Really Teaches Us
Lassa fever isn’t just a viral disease. It’s a mirror.
It reflects how infectious disease isn’t just about microbes — it’s about environment, inequality, and infrastructure. It reveals the gaps in healthcare systems, the vulnerability of those living closest to nature, and the consequences of scientific neglect. But it also shows us what’s possible when science, community, and policy begin working in sync.
We’ve seen how Lassa virus operates — from its microscopic structure to its macroscopic patterns of spread. We’ve traced it through homes, clinics, labs, and policymaker offices. And one thing becomes clear: Lassa is not unsolvable.
With recent diagnostic breakthroughs, better surveillance, real-world treatment trials, and the first serious vaccine candidates entering human testing, the scientific progress is undeniable. But it must be paired with systems that reach people where they are — not just in cities or headlines, but in rural villages, community health centers, and border towns.
More than anything, Lassa fever reminds us that the next outbreak is always one rodent, one missed diagnosis, one ungloved hand away. That’s the bad news — and the good news. Because once we know that, we can act. We can invest, educate, train, and prepare.
Lassa isn’t waiting for the world to catch up.
But maybe — just maybe — the world finally is.