Mpox (Monkeypox): Resurgence and Global Health Implications
Introduction
What Exactly Is Mpox?
You’ve likely heard the term “monkeypox” tossed around in news reports, especially in the wake of global health concerns over recent years. But what is it, really?
Mpox—yes, we’re using the updated name preferred by public health authorities to avoid stigma—is a viral zoonotic disease. That means it’s transmitted from animals to humans, a feature it shares with many of the more headline-grabbing infectious diseases of our time. But more technically, Mpox is part of the Orthopoxvirus genus. If that name rings a bell, you’re on the right track: this is the same viral family that includes the now-eradicated smallpox virus (Variola virus). In fact, the similarity between the two isn’t just taxonomical—it plays a big role in how we diagnose, treat, and even vaccinate against Mpox.
Think of Orthopoxvirus as a lineage of DNA viruses that are relatively large, complex, and historically formidable. While smallpox was officially declared eradicated in 1980, Mpox continues to circulate, especially in certain regions, and has been increasingly recognized as a pathogen of global significance.
A Brief History: Mpox Before the Spotlight
Mpox wasn’t born in a global pandemic. In fact, it was first identified in laboratory monkeys in 1958—hence the name “monkeypox”—although the name is misleading. Monkeys aren’t the natural reservoir of the virus. That role likely belongs to rodents, particularly African squirrels and pouched rats. The first human case wasn’t reported until 1970 in the Democratic Republic of the Congo, a few short years after smallpox was nearly on its last legs globally.
For decades, Mpox remained largely confined to parts of Central and West Africa. It was considered endemic—meaning it was consistently present but largely in the background. In these regions, outbreaks would flare up occasionally, often in connection with close contact with wildlife. The virus, while similar to smallpox, typically caused a milder illness, and it didn’t seem to spread easily between people.
So why the concern now? What changed?
From Regional Concern to Global Alarm
The early 2000s saw the first warning sign that Mpox was not as geographically limited as once thought. In 2003, the United States reported its first outbreak of Mpox, traced to imported African rodents that infected prairie dogs—yes, prairie dogs!—which were then sold as pets. Though no deaths occurred in that outbreak, it was a wake-up call: zoonotic viruses do not respect borders.
Fast forward to the early 2020s, and Mpox makes a dramatic re-entry onto the global stage. The 2022–2023 outbreaks stunned public health authorities—not because the virus had changed dramatically in terms of its genetic makeup (although mutations were noted), but because its transmission patterns had. Cases began appearing in countries with no history of Mpox, among populations without direct exposure to wildlife. For the first time, sustained human-to-human transmission in non-endemic regions became a reality.
Why Mpox Matters—Now More Than Ever
So, is Mpox the “next COVID”? Not exactly—and we need to be careful about falling into that comparison trap. But that doesn’t mean it’s harmless or that its resurgence should be taken lightly.
Mpox offers a real-time case study in how a neglected tropical disease can become a global health issue almost overnight. It challenges assumptions about viral containment, exposes gaps in surveillance and public health infrastructure, and underscores the interconnectedness of our modern world.
Fast forward to the early 2020s, and Mpox makes a dramatic re-entry onto the global stage. Much like Oropouche Fever, it reminded the world how a once-localized virus can quickly gain global relevance.
More importantly, Mpox is now a virus we must think about not just in terms of biology, but sociology, policy, ethics, and equity. Who gets vaccines? How do we communicate risks without stigmatizing affected communities? What are the economic and healthcare system implications of new outbreaks?
These aren’t theoretical questions. They are the kinds of urgent, practical inquiries we’ll be exploring in the sections to come.
Virology and Pathogenesis
What Does the Mpox Virus Actually Look Like?
When you hear “virus,” what comes to mind? A tiny spiky ball, maybe like the now-iconic image of the coronavirus? Mpox is… not that.
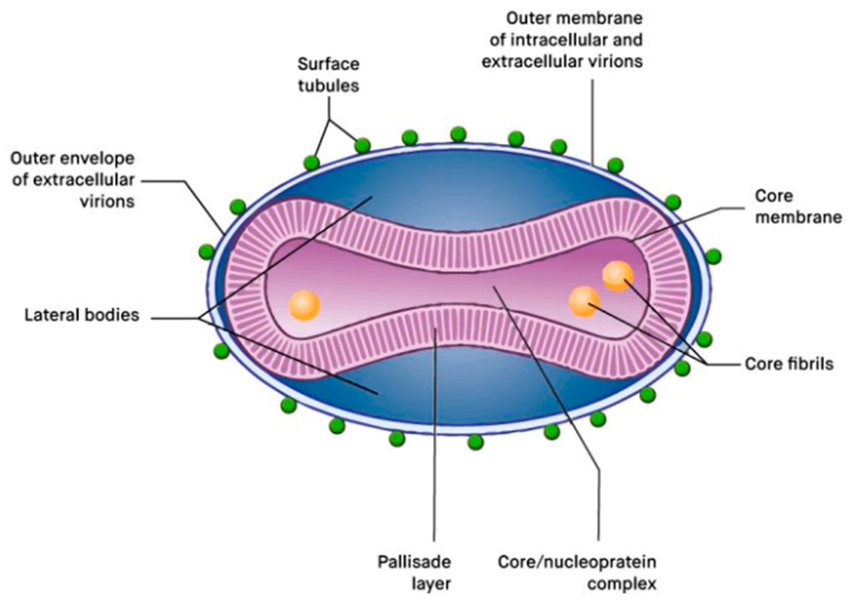
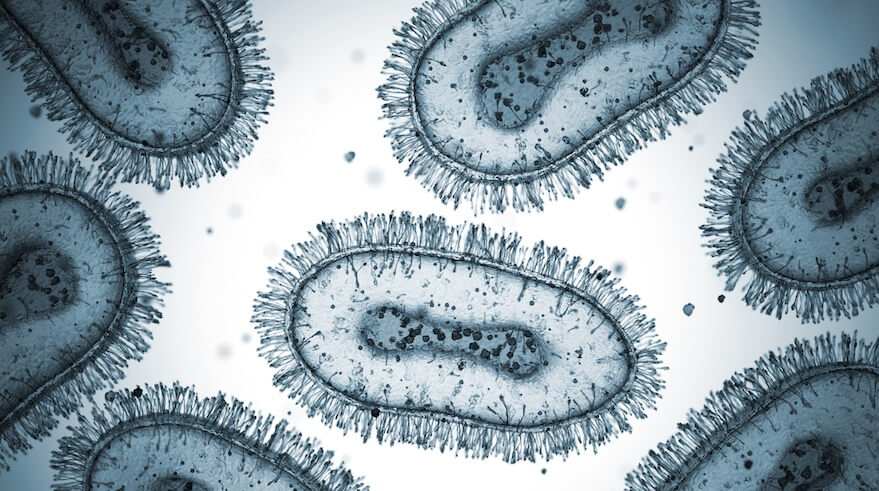
The Mpox virus is a brick-shaped, double-stranded DNA virus, which is a fancy way of saying it’s bigger and more complex than your average viral troublemaker. Under an electron microscope, it looks chunky—almost like a slightly flattened loaf of bread. It’s not trying to be cute, though; it’s a member of the Poxviridae family, specifically the Orthopoxvirus genus, making it a close relative of smallpox, vaccinia (used in vaccines), and cowpox viruses.
Unlike most DNA viruses, which tend to replicate inside the nucleus of a host cell (where all the genetic goodies are), Mpox is one of the few that sets up shop in the cytoplasm—the jelly-like stuff outside the nucleus. This is no small feat. It means the virus carries a lot of its own molecular machinery, giving it a kind of “do-it-yourself” attitude when it comes to replication. It doesn’t need to borrow as much from the host cell because it’s already packed with enzymes and proteins that allow it to replicate independently.
The Mpox Genome: A Blueprint for Trouble
Mpox’s genome is large—over 190,000 base pairs long—which is pretty bulky in viral terms. That’s like comparing a full novel to a short poem in the viral literary world. This expansive genome allows it to encode for more than 200 proteins, many of which are devoted not just to replication but to evading the immune system.
Think of it this way: some viruses are stealthy, sneaking past the immune system before it even notices them. Mpox isn’t subtle—it walks in with a strategy. Its proteins actively interfere with immune signaling, disrupt the host’s ability to mount a strong response, and even block certain inflammatory pathways. This makes it harder for your body to clear the infection quickly and contributes to the symptoms we’ll discuss in more detail later.
How Does It Infect You?
Let’s say someone comes into contact with the virus—either through close personal contact, a contaminated surface, or possibly respiratory droplets (though this is less common). What happens next?
The virus typically enters through broken skin, the respiratory tract, or mucous membranes. Once inside, it targets epithelial cells—the ones lining your skin, respiratory tract, and internal organs. This is where the infection begins, usually at the point of entry, before the virus migrates to local lymph nodes.
From there, it spreads via the bloodstream in a process known as primary viremia. At this stage, the immune system starts catching on, sending out signals like interferons and cytokines—its version of a general alarm. But Mpox, as we’ve seen, is prepared. It pushes back against this response, and the result is a second wave of viral replication in the skin, leading to the characteristic rash and lesions.
Interestingly, this pattern of spread—initial replication, lymph node involvement, followed by a secondary skin-centric phase—mirrors that of smallpox. The immune system recognizes these poxviruses in very similar ways, and that’s part of why the smallpox vaccine offers cross-protection against Mpox (more on that in the prevention section).
The Immune Response: Defender and Damage-Maker
So how does your body respond? Well, it depends on your overall health, vaccination status, and even your genetics. But in general, the immune response involves both innate and adaptive immunity.
The innate immune system is the first to react. It’s fast, but not very specific. Think fever, inflammation, and general malaise. Natural killer cells and macrophages are deployed like front-line soldiers.
The adaptive immune system kicks in later. This is your precision force—T-cells that kill infected cells and B-cells that produce virus-neutralizing antibodies. This is also where memory comes into play: if you’ve been vaccinated against smallpox, your body may recognize Mpox more quickly and respond more efficiently, often leading to a milder illness or preventing it altogether.
However, in some people—especially those who are immunocompromised or unvaccinated—the virus can overpower these defenses, leading to more serious disease.
Does the Virus Mutate Like Other Pathogens?
Here’s where things get interesting. Mpox is a DNA virus, which means it generally mutates much more slowly than RNA viruses like influenza or SARS-CoV-2. That’s because DNA replication includes built-in error correction—essentially proofreading enzymes that fix mistakes before they become permanent mutations.
But—and it’s a big but—recent outbreaks have shown more genomic variation than expected. Researchers are investigating whether this is due to human-to-human transmission under new conditions or some kind of viral adaptation. Could Mpox be evolving more rapidly in response to selective pressures? It’s an active area of research, and it underscores the importance of ongoing genomic surveillance.
This microscopic warrior may not be as famous as its cousin smallpox, but Mpox has its own arsenal and ambitions. In the next section, we’ll zoom out from the cellular battlefield and look at the big-picture trends: Where is Mpox spreading? Who is most affected? And how are these patterns shifting in real time?
Epidemiology
Where in the World Is Mpox?
For decades, Mpox felt like a regional concern—something “over there” in parts of Central and West Africa. And to be fair, that’s where it first emerged and where it has persisted most reliably. Countries like the Democratic Republic of the Congo, Nigeria, Cameroon, and the Central African Republic have seen repeated outbreaks, some small and localized, others larger and more sustained. In these areas, Mpox has long been considered endemic, meaning the virus circulates regularly within the population, typically jumping from animals to humans.
But if you’re reading this from outside Africa, chances are you didn’t start paying attention to Mpox until fairly recently—and you wouldn’t be alone. It wasn’t until 2022 that Mpox truly exploded into the global consciousness. Cases began appearing in Europe, North America, South America, and parts of Asia, seemingly out of nowhere.
And that’s what made public health experts sit up straight.
The 2022–2023 outbreaks were not tied to travel from endemic regions in many cases. Instead, they involved sustained local transmission in communities that had never seen Mpox before. It became clear that this wasn’t just a spillover from animals. Human-to-human spread was happening, and it was happening in countries with robust healthcare infrastructure.
So today, Mpox isn’t just a tropical zoonosis—it’s a global infectious disease threat. And that shift has changed how we need to think about surveillance, preparedness, and public messaging.
Who’s Getting Infected?
Traditionally, Mpox was mostly a pediatric disease. In endemic regions, the bulk of cases were in children, often in rural areas with close contact to wildlife. But the recent outbreaks have rewritten that demographic script.
In the 2022–2023 waves, the majority of reported cases occurred in young to middle-aged adults, particularly men who have sex with men (MSM). This wasn’t about blame or moral judgment—it was about epidemiology. Close, prolonged physical contact, including sexual contact, turned out to be a major transmission pathway. Skin-to-skin interaction, especially with broken skin or mucous membranes, proved incredibly efficient for spreading the virus.
But here’s where nuance matters: Mpox is not a “sexually transmitted infection” in the classic sense, like gonorrhea or HIV. It’s not limited to sexual transmission, and anyone—regardless of sexual orientation—can get infected through close contact, contaminated materials (like bedding or towels), or respiratory droplets in certain settings. Focusing too narrowly on one community risks missing the broader picture—and worse, can stigmatize those most at risk.
That said, understanding who is getting infected helps tailor outreach, prioritize vaccine distribution, and build culturally sensitive health campaigns. It’s not about assigning fault; it’s about understanding transmission so we can stop it.
How Does Mpox Spread?
This is the million-dollar question, and the answer is evolving.
Traditionally, Mpox was believed to spread primarily through:
- Animal-to-human contact, such as bites, scratches, or handling bushmeat
- Human-to-human contact, via broken skin, mucous membranes, or respiratory droplets in prolonged face-to-face contact
- Fomites—objects or surfaces contaminated with the virus (think bedding, clothing, or shared towels)
In recent outbreaks, skin-to-skin contact emerged as the dominant transmission route, particularly during intimate encounters. But respiratory spread, while less common, still matters—especially in crowded indoor settings or healthcare environments.
One big shift is that asymptomatic or mildly symptomatic carriers may now be playing a role in spread. That wasn’t previously thought to be significant. But if someone has only a few subtle lesions—or lesions hidden in areas like the genital or perianal region—they might not realize they’re contagious.
This has made containment harder. Unlike smallpox, which was visually obvious and had a clear symptom profile, Mpox can be deceptively mild, especially in vaccinated individuals or people with partial immunity.
Risk Factors: It’s Not Just Geography Anymore
So who’s most at risk? Well, let’s broaden the lens.
- Healthcare workers, especially those handling suspected cases without proper protective equipment, are at elevated risk—though guidelines and training have helped reduce exposure.
- People with compromised immune systems—whether due to HIV, cancer treatments, or organ transplants—may be more susceptible to severe disease and complications.
- Unvaccinated individuals, especially younger people who were born after routine smallpox vaccination ended (in the 1980s for most countries), are now a growing cohort of vulnerability.
Also important: social determinants of health. People in overcrowded housing, those with limited access to healthcare, or members of marginalized communities may face both higher exposure risk and fewer resources for prevention or treatment. In other words, Mpox exploits cracks in the system.
Is Mpox Seasonal or Year-Round?
Mpox doesn’t follow a strict seasonal pattern like flu or RSV, but in endemic regions, cases do tend to spike in certain months—often tied to rainy seasons and increased animal activity. In newly affected countries, seasonality isn’t well understood yet, though large social gatherings (festivals, parties, Pride events) have been linked with case spikes, suggesting that behavioral rhythms may matter more than climate for now.
This opens a door for proactive prevention: If we know when people are most likely to be in close contact, we can plan awareness campaigns, vaccine rollouts, and surveillance efforts accordingly.
Mpox is no longer content to stay in the shadows of equatorial Africa. It’s global now, and its epidemiology is shifting under our feet. Understanding where it’s spreading and who it affects is key to stopping it in its tracks.
Next up, we’ll explore what happens after infection begins—Part Four: Clinical Manifestations. How does Mpox show up in the body? What symptoms should you look for? And how severe can it get?
Clinical Manifestations
What Does Mpox Feel Like?
If you’ve been exposed to Mpox—or are treating someone who has—you’re likely wondering: What does this virus actually do? How does it present?
The short answer: it’s often described as “smallpox-lite,” but that undersells the range and complexity of Mpox’s clinical picture.
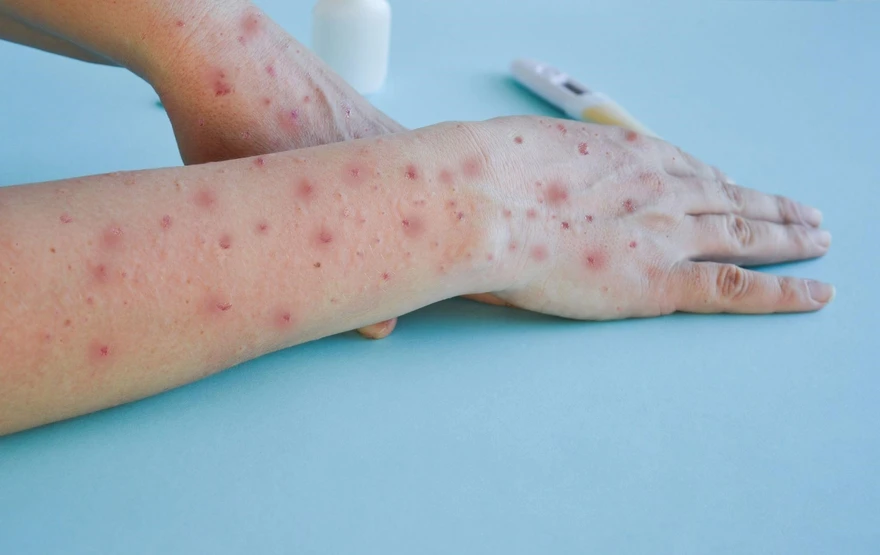
After an incubation period of about 5 to 21 days (the average is closer to 7–14 days), symptoms begin to appear. This delay can be frustrating, especially for contact tracing—because by the time symptoms show up, the person may have already transmitted the virus. But once the illness starts, it usually unfolds in two distinct phases.
Phase One: The Invasion Period
This is the part that can feel like the flu. It typically lasts 1 to 4 days and is marked by:
Fever
Almost universally present at onset. Temperatures can spike high, and it’s usually one of the first clues that something’s wrong.
Intense Headache
Not your garden-variety tension headache. People often describe it as deep, pounding, and hard to relieve.
Lymphadenopathy (Swollen Lymph Nodes)
This one matters. Swollen lymph nodes—especially in the neck, armpits, or groin—are a hallmark feature that helps distinguish Mpox from other pox-like illnesses, such as smallpox or chickenpox.
Myalgia and Fatigue
Body aches, chills, back pain, and exhaustion round out the invasion period. These symptoms reflect your immune system in high gear, battling the initial wave of viral replication.
Then comes the second phase—the one that’s hard to miss.
Phase Two: The Skin Breaks Out
A few days after the fever starts, a rash appears. And not just any rash. It typically begins on the face (in over 95% of cases in classical outbreaks), then spreads centrifugally to the palms, soles, arms, legs, and eventually the trunk.
But here’s where things get clinical—and graphic.
The rash progresses in a well-defined sequence, almost like a time-lapse of viral warfare:
- Macules – flat, red spots
- Papules – raised bumps
- Vesicles – fluid-filled blisters
- Pustules – those blisters fill with pus
- Scabs – the pustules dry, crust, and eventually fall off
Each lesion goes through the full evolution before resolving, which can take 2 to 4 weeks. Unlike chickenpox, where you might see spots at different stages all at once, Mpox lesions tend to be synchronous—they develop and resolve together in the same area.
Does It Always Look Like That?
Not anymore.
One of the more surprising aspects of recent global outbreaks is that the clinical picture has shifted. Instead of a full-body rash, some people—especially those infected through intimate contact—have localized lesions, particularly in the genital, perianal, or oral regions. In some cases, these may be the only visible signs of infection.
This subtler presentation can make diagnosis harder. It also explains why Mpox has sometimes been mistaken for other sexually transmitted infections, like herpes or syphilis. That’s a major challenge for frontline clinicians and a reason why clinical suspicion must remain high, especially when evaluating at-risk individuals.
What About Pain? Is Mpox Mild or Miserable?
That depends. While the illness is often self-limiting, meaning it resolves on its own without specific treatment, that doesn’t mean it’s easy.
- Lesions can be excruciating, especially in mucosal areas (mouth, genitals, rectum).
- Some patients experience proctitis—inflammation of the rectum—which can cause intense pain, bleeding, and difficulty passing stool.
- Others develop secondary infections in skin lesions, which can worsen the course and prolong recovery.
In hospitalized cases, pain management becomes a priority. The physical discomfort can rival that of shingles or even burn injuries in some instances.
Who’s at Risk for Severe Disease?
For most healthy adults, Mpox is a miserable but manageable illness. However, certain groups are more likely to experience complications:
- Young children, particularly those under 8 years old
- Pregnant people, due to risks of fetal infection or pregnancy complications
- Immunocompromised individuals, such as those with uncontrolled HIV/AIDS or those on immunosuppressive drugs
In these populations, Mpox can cause systemic complications such as:
- Secondary bacterial infections (cellulitis, sepsis)
- Pneumonia
- Encephalitis (inflammation of the brain)
- Corneal infections, which can lead to vision loss
The mortality rate in endemic African outbreaks has historically varied from 1% to 10%, depending on the viral clade and the health status of the affected population. Fortunately, the clade responsible for the 2022–2023 global outbreaks (Clade IIb) appears to be less lethal than the more virulent Clade I found in Central Africa.
Is Reinfection Possible?
It’s rare, but not impossible. Most people develop a strong immune response after infection, which likely offers some degree of long-term protection. However, because surveillance data is still limited—and because the virus may be evolving slightly—we don’t have definitive answers about lifelong immunity just yet.
Patients have come in with: just a few discreet lesions (sometimes internal), symptoms resembling sexually transmitted infections, or no rash at all — diagnostic ambiguity that, like the situation around evolving diagnostic criteria in Autism in 2025, demands constant clinical recalibration.
Mpox isn’t just a rash. It’s a systemic illness that can range from uncomfortable to life-threatening. Recognizing the full spectrum of clinical manifestations is essential—not just for early treatment, but for reducing stigma and preventing further spread.
Diagnosis
How Do You Know It’s Mpox?
Seems like a simple question, right? There’s a rash, maybe a fever, some swollen lymph nodes — just run a test and call it a day. But as with many infectious diseases, diagnosing Mpox is less clear-cut than it sounds, especially in the early stages or in mild cases.

The challenge is that Mpox’s symptoms overlap with a lot of other conditions. Think about it: fever, headache, fatigue — could be flu, COVID, even dengue or malaria in some parts of the world. And the rash? That’s a tricky one. It might look like chickenpox, herpes, syphilis, or even a drug reaction, depending on how and where it appears.
So how do clinicians make the call? And more importantly, how do labs confirm it?
The Gold Standard: PCR Testing
At the heart of modern Mpox diagnosis is the polymerase chain reaction, or PCR. You’ve probably heard of it in the context of COVID-19 — same concept, different target.
PCR works by detecting the virus’s DNA in a clinical specimen — usually a swab taken from one of the patient’s skin lesions. And here’s why that matters: Mpox, like all Orthopoxviruses, has a double-stranded DNA genome, which makes it a great candidate for PCR detection. DNA is more stable than RNA (what coronaviruses use), so it tends to stick around longer and degrade more slowly, giving labs more to work with.
But PCR testing isn’t just about saying “yes or no.” A well-designed assay can even differentiate between Orthopoxviruses, telling you whether it’s Mpox, vaccinia (used in vaccines), or even smallpox (in the extremely unlikely event that turns up). That said, many labs initially rely on general Orthopox detection, and only specialized reference labs can do full genomic differentiation.
Sample Collection: Why Lesions Matter
Timing and technique are key.
PCR is most reliable when it’s run on skin lesion material — ideally swabs from the surface of a pustule, or the fluid within a vesicle. Scab material can also work, but early in the infection, when the rash is just starting or hasn’t yet appeared, things get tricky.
If a person is still in the invasion phase (remember: fever, headache, swollen nodes, no rash yet), there might be no visible lesions to test. In such cases, clinicians might collect throat swabs, blood, or even rectal swabs in certain populations. But those samples are less reliable, with higher chances of false negatives.
The bottom line? If you suspect Mpox but have no lesions to sample, you’re in a diagnostic gray zone. And that’s why…
Early Diagnosis Is Hard
In the recent outbreaks, clinicians across the globe have run into a common frustration: Mpox doesn’t always look like Mpox.
Patients have come in with:
- Just a few discreet lesions (sometimes internal, such as in the rectum or throat)
- Symptoms resembling sexually transmitted infections
- No rash at all, only fever or pain
- Negative PCR results early in infection
This can delay both diagnosis and isolation, increasing the risk of further spread — especially in urban areas or congregate settings. And in some countries, stigma or lack of provider familiarity has made diagnosis even more difficult.
There’s also the challenge of access. Not every clinic has the capability to collect, store, and ship samples to specialized labs. In resource-limited settings — including many areas where Mpox is endemic — PCR may simply be unavailable. That leads to underreporting, misdiagnosis, or treating based on clinical suspicion alone.
Serology: Can Antibodies Help?
In theory, yes. Serological testing looks for antibodies — proteins your immune system produces in response to the virus. This can tell you whether someone has been infected, even if they’ve already recovered or were asymptomatic.
But there are caveats. First, antibodies don’t appear immediately. It can take 1–2 weeks or more for IgM and IgG levels to become detectable. That makes serology less useful for diagnosing active cases.
Second, Orthopoxviruses are antigenically similar — meaning they trigger cross-reactive antibodies. If you had a smallpox vaccine as a child, or were exposed to cowpox or vaccinia, you might have antibodies that muddy the result. So while serology can be helpful for retrospective studies or confirming past exposure, it’s not the frontline tool for acute diagnosis.
What About Rapid Tests?
We’re not quite there yet.
While there’s a push to develop point-of-care diagnostics — quick, cheap tests that could be used outside of labs — we’re still in the early stages. A handful of prototypes and field-deployable PCR systems exist, but widespread use remains limited by cost, infrastructure, and validation hurdles.
In short, we don’t yet have a “Mpox rapid test” like we do for COVID or flu. That’s a glaring gap — and one that the global health community is actively trying to fill.
Imaging and Other Tools?
In severe or atypical cases, additional tools might come into play.
- CT or MRI scans can assess complications like encephalitis or proctitis.
- Colonoscopy has even been used in some patients with intense rectal pain or bleeding.
- Dermatoscopy (visual magnification of skin lesions) may help in differential diagnosis, though it’s more helpful for dermatologists than frontline clinicians.
Still, these are supporting tools, not diagnostic cornerstones.
Diagnosing Mpox is part science, part timing, and part clinical intuition. PCR testing is our best friend—but even it has blind spots. That’s why a high index of suspicion, clear communication with patients, and well-trained clinicians are just as important as the test itself.
Treatment and Management
So You’ve Diagnosed Mpox… Now What?
Let’s imagine the test comes back positive. Or maybe the clinical picture is clear enough that a diagnosis is made even before lab confirmation. The question is immediate: What now?
Here’s the truth: while Mpox may sound like a modern viral threat, it’s still being managed with some decidedly old-school tools — rest, fluids, symptom control. There’s no silver bullet, no one-size-fits-all pill that knocks it out. But that doesn’t mean we’re helpless.
Mpox management today is a blend of supportive care, targeted symptom relief, risk stratification, and — in selected cases — antiviral therapy. And yes, vaccine-derived immunity plays a quiet but crucial backstage role too, especially in preventing severe illness.
Let’s unpack what that actually looks like in practice.
Supportive Care: The Foundation of Treatment
Most cases of Mpox are self-limiting, meaning the body will clear the virus on its own within 2 to 4 weeks. So for the majority of patients — especially healthy adults — treatment is about managing symptoms and avoiding complications.
That sounds simple, but it can be deceptively intensive. Consider what “supportive care” really involves:
- Pain control is key. Lesions, especially in mucosal areas like the mouth or genitals, can be agonizing. Think about how uncomfortable a single cold sore can be — now imagine dozens. Providers often turn to NSAIDs, acetaminophen, and in severe cases, even prescription-strength analgesics or topical anesthetics.
- Hydration and nutrition matter. Patients with oral lesions may struggle to eat or drink. Some may require IV fluids, or, in rare cases, temporary feeding support to prevent dehydration and malnutrition.
- Skin care is vital for preventing secondary bacterial infections. Gentle cleansing, antiseptic soaks, and appropriate dressings can help protect the integrity of broken skin and promote healing.
- Mental health support can’t be overlooked. Mpox isn’t just physically painful — it’s also isolating. Patients may be quarantined for weeks, dealing with visible lesions, discomfort, and fear. Anxiety and depression are common. Compassionate care means treating the whole patient, not just the virus.
When Do You Reach for Antivirals?
Mpox and smallpox are close relatives, and that biological similarity is what gives us an edge — because while we don’t have drugs developed specifically for Mpox, we do have smallpox antivirals that offer a path forward.
The most prominent player? Tecovirimat (TPOXX).
Originally developed to treat smallpox under bioterrorism preparedness programs, tecovirimat targets a viral envelope protein that Orthopoxviruses need to exit infected cells. In other words, it stops the virus from spreading within the body.
Does it work for Mpox? Evidence suggests yes — but cautiously so. While animal studies and compassionate use data are promising, we still lack large-scale randomized clinical trials. That said, tecovirimat has been widely used during the recent outbreaks, especially in:
- Patients with severe disease
- Those with mucosal or ocular involvement
- Immunocompromised individuals
- Children or pregnant people at higher risk of complications
It’s generally well tolerated, taken orally for 14 days, and made available under expanded access or emergency use protocols in many countries.
What About Other Antivirals?
Two other candidates occasionally come up:
Cidofovir
An older antiviral originally used for cytomegalovirus. It has some activity against Orthopoxviruses, but it’s nephrotoxic(can damage the kidneys), and must be administered intravenously. Not ideal for routine use.
Brincidofovir
A lipid-conjugated version of cidofovir that’s easier on the kidneys and available orally. It seemed promising at first, but early studies in Mpox didn’t show much clinical benefit. Research continues, but it’s currently used only in specific cases.
Investigational and Adjunctive Therapies
What if a patient is too sick for oral antivirals? What if they can’t swallow or absorb meds, or develop complications beyond skin and mucosa?
In these scenarios, IV tecovirimat may be considered (still under investigation in most settings). Some clinicians have also used vaccinia immune globulin (VIG) — a pooled antibody therapy derived from vaccinated individuals — though this is typically reserved for immunocompromised patients or those with vaccine-related complications.
Then there’s experimental terrain: monoclonal antibodies, combination therapies, and novel antivirals are all under study. But for now, the best tools remain the ones we already have — used wisely, compassionately, and with clinical judgment.
Managing Special Populations
Some patients require tailored approaches:
- Pregnant individuals face unique risks — Mpox can cause fetal loss, congenital infection, or complications during delivery. Management involves balancing maternal treatment with fetal safety. Antivirals may be offered under careful supervision.
- Children, particularly under age 8, are at greater risk of complications and may need hospitalization for close monitoring and supportive care.
- People with HIV, especially if untreated or with low CD4 counts, may experience prolonged illness or unusual manifestations. These patients often benefit from antiviral therapy and careful follow-up.
The Role of Isolation and Containment
Treating Mpox also means stopping it from spreading. That’s why isolation remains a core management principle. Patients are usually advised to stay isolated at home or in designated facilities until all lesions have crusted, scabs have fallen off, and new skin has formed — a process that can take weeks.
Healthcare providers must use PPE (gloves, gowns, eye protection, and respirators in certain cases) when managing Mpox patients. Household contacts should be monitored, and in some cases, offered post-exposure prophylaxis with vaccine (we’ll cover that in the next section).
In many ways, treating Mpox today feels like navigating a tightrope between what we do know from smallpox medicine and what we’re still learning in real time. It’s not flashy, and it’s not always straightforward. But with good symptom control, targeted antivirals, and a deep respect for each patient’s context, we can do a lot — even without a miracle cure.
Prevention and Control
Can We Really Prevent Mpox?
Let’s not dance around it — yes, Mpox can be prevented. But like most things in public health, it’s not just a matter of having the right tools. It’s also about using them in the right way, at the right time, for the right people.
At its core, Mpox is not an invisible enemy. It spreads primarily through close physical contact — skin-to-skin, face-to-face, or by handling contaminated materials. That gives us an edge: this isn’t an airborne ghost like measles or COVID. It’s physical, tangible, and relatively slow-moving. In theory, that should make it easier to contain.
And yet, the story has been anything but simple.
In the earlier, more geographically limited outbreaks, Mpox stayed mostly within the borders of Central and West African countries. The virus passed quietly from animals to humans — often through hunting, butchering, or preparing bushmeat — and occasionally from one person to another within households or small communities. Over time, public health messaging in these regions emphasized hygiene, animal handling precautions, and recognition of early symptoms. For a while, that seemed like enough.
But then, the pattern changed.
With the 2022–2023 outbreaks, Mpox began showing up in large urban centers and global cities, far from the forests of the Congo Basin. What changed wasn’t the virus’s basic structure — it was its social context. Now, transmission was often happening in intimate settings between people with no known exposure to animals. Some cases emerged after parties or festivals. Others were linked to close-contact environments like gyms or shared housing. This forced public health authorities to rethink what prevention really means in a hyperconnected world.
What they found is that knowledge — real, practical knowledge — is just as important as biology.
Preventing Mpox now means educating people without shaming them. It means communicating how the virus spreads while avoiding the dangerous trap of stigmatizing the communities most affected. During recent outbreaks, that meant talking openly about sexual transmission without turning the illness into a “gay disease.” It meant explaining that Mpox is not technically an STI, but that close intimate contact is still the highest-risk context for spread. Public health teams had to thread a needle: alerting at-risk groups without turning them into scapegoats.
Of course, awareness only goes so far. Prevention also depends heavily on vaccines — and here, we’re in a uniquely fortunate position. Thanks to decades of smallpox research and bioterrorism preparedness, we already had vaccines in the freezer. Mpox and smallpox are viral cousins, close enough genetically that the same vaccines can offer cross-protection. That gave us a huge head start.
JYNNEOS, a non-replicating, next-generation vaccine developed originally for smallpox, emerged as the frontrunner in the Mpox response. It’s safe, effective, and suitable even for immunocompromised individuals. Most importantly, it can be used both before and after exposure. During outbreaks, many people received the vaccine as post-exposure prophylaxis, especially if they were household contacts or had prolonged intimate contact with confirmed cases. For those at higher ongoing risk — such as some healthcare workers or individuals in certain sexual networks — pre-exposure vaccination has been recommended in several countries.
And yet, despite all this scientific readiness, one stubborn problem has refused to go away: inequity.
The global response to Mpox laid bare the same uncomfortable truth we saw with COVID — that vaccine access is still lopsided. The regions where Mpox has existed the longest, and where people live with the highest baseline risk, are often the last to receive meaningful vaccine shipments. Central and West African nations, despite sounding the alarm about Mpox for decades, have frequently been excluded from the vaccine distribution priority lists. Meanwhile, wealthier countries scrambled to secure stockpiles once the virus appeared in their cities.
This isn’t just a logistical oversight; it’s a moral failing. Global prevention depends on global fairness. You can’t contain a virus by building fences around wealthy countries. You have to strengthen the foundations everywhere, especially where the threat has always been present.
Even the most advanced vaccines and treatments won’t matter if they’re stuck in warehouses or withheld behind patent walls. Fortunately, international coalitions are beginning to respond. The World Health Organization, GAVI, and several public-private partnerships are now working to ensure broader access, but progress is uneven and trust remains fragile. In many places, historical wounds still shape how public health efforts are received — especially when they’re seen as parachuted in during emergencies but absent the rest of the time.
Prevention isn’t just about the virus. It’s about the people. It means supporting communities with accurate information, ensuring they have access to tools that work, and investing in public health systems that can do the job long after the headlines fade. That includes training local healthcare workers, maintaining robust surveillance networks, and integrating Mpox awareness into broader health outreach — not as a one-time campaign, but as part of routine care.
In that sense, Mpox may not just be a viral threat. It might be a test of our readiness — not just for the next virus, but for the world we want to build in its wake. Just as lessons from historic outbreaks like the Plague echo forward, so too must our strategies adapt to modern realities.
In the end, preventing Mpox comes down to one principle: we already know how. We just have to choose to act on that knowledge with courage, compassion, and equity.
And as we’ll see in the next section, Recent Developments (2025–2026), the story is still unfolding. Outbreaks are continuing, new tools are emerging, and the virus — and our response to it — is still evolving.
Recent Developments (2025–2026)
Is Mpox Still a Global Concern in 2025?
If you’re wondering whether Mpox is still relevant — whether it’s faded from the spotlight or remains a lingering threat — the answer is more layered than a simple yes or no. In some places, Mpox is already becoming part of the new normal: monitored, managed, and occasionally flaring. In others, it’s an urgent reminder that outbreaks don’t need novelty to be dangerous — just enough indifference.
Over the past year, the global profile of Mpox has shifted once again. While the initial wave of international panic has receded, the virus hasn’t disappeared. Instead, it has found pockets of persistence — clusters of cases in both familiar and unexpected places. And as we’ve learned (often the hard way) with infectious diseases, what’s quiet today can roar tomorrow if conditions align.
Where Are the Outbreaks Happening Now?
Since late 2024, public health surveillance systems — much improved from the disjointed early days — have detected several notable Mpox outbreaks. Some have occurred in previously affected regions, like parts of West Africa and Brazil, while others have emerged in new urban centers, including a surprising uptick in southern Europe and Southeast Asia.
One recurring pattern? Localized flare-ups tied to dense social events or gatherings. While the virus isn’t airborne in the traditional sense, the intimacy of festivals, nightlife scenes, and transient housing creates just enough friction for Mpox to find a foothold. In one recent case study from Barcelona, public health officials traced over 200 cases back to a week-long event where vaccination coverage was patchy and messaging hadn’t penetrated far enough beyond the core at-risk groups.
In Central Africa, where Mpox has never really left, surveillance has become more targeted and proactive. Thanks to support from international partners, digital tools now assist with case tracking and real-time alerts — but logistics and funding remain uneven, especially in rural areas. Some provinces are seeing a drop in cases; others are quietly escalating.
Are We Diagnosing Faster?
Yes — but there’s still room to grow. One of the biggest wins of the last 12 months has been the acceleration of diagnostic capacity, particularly in low- and middle-income countries. Regional labs in Africa, Latin America, and parts of Asia now have more consistent access to PCR testing equipment and trained technicians, allowing them to confirm cases more quickly and with fewer false negatives.
What’s changed isn’t just the technology — it’s the integration. Mpox testing is no longer siloed; it’s increasingly part of routine febrile illness panels in hospitals and clinics. This means someone presenting with unexplained rash or fever might get tested for Mpox alongside dengue, HIV, and even syphilis — a diagnostic approach that reflects the realities of overlapping symptoms and co-infections.
There’s also been steady, though cautious, progress in point-of-care tests. A few lateral flow assays — similar to rapid COVID tests — are now in pilot deployment. They aren’t perfect, but in field settings where lab access is days away, a decent rapid test can buy time and guide urgent care decisions.
What’s New in Treatment?
Treatment options haven’t changed dramatically, but they’ve matured. Tecovirimat remains the cornerstone for severe or high-risk cases, and there’s now a clearer global framework for accessing it through expanded use protocols. Many countries have established tiered guidelines: mild cases stay home with supportive care; moderate cases are triaged for antivirals based on comorbidities; and severe cases receive priority access with specialized clinical monitoring.
What’s changed is the refinement of dosing strategies and risk assessment, especially for vulnerable populations like children and pregnant individuals. For instance, several case series published in early 2025 have helped define how to safely use antivirals in pediatric settings — something that was largely guesswork during the initial waves.
Meanwhile, a few new antiviral candidates are entering Phase II trials. These include compounds that target viral entry proteins or enhance the immune system’s response without triggering excess inflammation. We’re still at the early stages here — but the pipeline, once almost empty, is now cautiously humming with promise.
And Vaccines?
Here’s where we’re seeing real movement.
JYNNEOS remains the workhorse, but now it’s joined by a second-generation candidate called PXVX-MPX, developed specifically for Mpox rather than adapted from smallpox. Early data suggest a stronger and more sustained antibody response after a single dose — potentially game-changing in settings where completing a two-dose schedule is a logistical nightmare.
Some regions are experimenting with ring vaccination plus community rollout, a hybrid strategy designed to create “firebreaks” around known clusters while also reaching high-risk groups more broadly. This tactic has shown promise in containing flare-ups quickly, without needing full-scale mass vaccination — a nod to both science and realism.
Importantly, vaccine outreach has improved. Community-based organizations, especially in LGBTQ+ and migrant communities, have become partners rather than passive recipients. In several cities, Mpox vaccine pop-ups are now embedded in broader sexual health services — a small but powerful shift toward trust and accessibility.
What’s on the Horizon?
Perhaps the most important change since 2022 isn’t in the virus — it’s in how we think about Mpox.
We’re no longer framing it as an exotic zoonosis or a global fluke. It’s being treated as what it is: a persistent, adaptive threat that can be managed, but not ignored. Public health curricula have updated. Clinical protocols have been revised. Mpox has carved out a place in the ongoing dialogue around emerging infectious diseases, alongside Marburg, H5N1, and others that tend to lurk just beyond the edges of public attention.
And in a broader sense, Mpox is teaching us how to respond without panic — to build infrastructure for the long haul, rather than sprint from crisis to crisis. It’s a test of our ability to learn from past failures and invest in health systems that work even when headlines fade.
Frequently Asked Questions (FAQ)
1. Is Mpox the same as smallpox?
Not exactly. Mpox and smallpox are closely related — both are Orthopoxviruses — but they are different diseases. Smallpox was far more lethal and was eradicated globally in 1980. Mpox is typically milder, with a lower mortality rate, but it still poses serious health risks, especially for certain vulnerable populations. Importantly, the smallpox vaccine offers some protection against Mpox because of their genetic similarity.
2. Can Mpox be sexually transmitted?
Mpox is not classified as a traditional sexually transmitted infection (STI), but sex — especially involving close skin-to-skin or mucosal contact — is a common setting for transmission. It’s the physical closeness, not the sexual act itself, that facilitates spread. Recent outbreaks have shown that intimate contact, especially among networks of close physical interaction, can be a major driver of new cases.
3. What does Mpox look like and feel like?
It often starts with fever, headaches, swollen lymph nodes, and fatigue — a kind of viral “prodrome.” Then comes a distinctive rash that evolves from flat spots to raised bumps, then into fluid- and pus-filled lesions that eventually scab over. The rash can be localized or widespread, and in many recent cases, lesions have appeared primarily in the genital or perianal areas. The illness usually lasts 2 to 4 weeks.
4. How do doctors test for Mpox?
The gold standard is PCR testing, usually from a swab taken from a skin lesion. Other samples like throat or rectal swabs may be used in early cases, but they’re less reliable. Rapid tests are in development but not yet widely available. Serology (antibody tests) can show past exposure but isn’t helpful for confirming active cases.
5. Is there a treatment for Mpox?
Most cases are treated with supportive care — managing pain, preventing dehydration, and ensuring good wound care. For more severe cases, or people at higher risk (like immunocompromised individuals), an antiviral called tecovirimat (TPOXX) may be used. It’s not a cure, but it can shorten the illness and reduce complications.
6. Can you get vaccinated against Mpox?
Yes. The JYNNEOS vaccine (also called Imvanex or Imvamune) offers protection and is used for both pre-exposure and post-exposure scenarios. It’s derived from the vaccinia virus but does not replicate in the human body, making it safer for people with weakened immune systems. An older vaccine, ACAM2000, is more immunogenic but carries higher risks and is used more selectively.
7. Is Mpox still spreading in 2025?
Yes, though at a more manageable pace. Outbreaks are still occurring in parts of Africa, Europe, and South America, often linked to specific social settings. Surveillance and response efforts have improved, and vaccine strategies are being more carefully and equitably deployed.
8. Can Mpox come back after recovery?
Reinfection appears rare, but long-term data is limited. Most people develop a strong immune response after infection, especially if they receive vaccination as part of their post-infection care. However, like with many viruses, our understanding of immunity is still evolving.
Conclusion and Future Directions
So where does this leave us?
Mpox began as a low-profile zoonotic disease, mostly ignored outside the countries where it quietly circulated for decades. But in the last few years, it’s forced its way into the global conversation — not with the shock-and-awe of a novel pandemic, but with the unsettling steadiness of a virus that knows how to wait.
It revealed weaknesses in our public health systems — in how we monitor diseases, how we talk about risk, how we prioritize prevention before panic. But it also showed what’s possible when we respond thoughtfully. Mpox is no longer “forgotten.” It’s studied, tracked, and, increasingly, managed.
Still, vigilance matters. This is a virus with the potential to expand, adapt, and reemerge in places we don’t expect. Whether through travel, social contact, or environmental shifts, the conditions for resurgence are always one step away. And yet, unlike some threats, Mpox offers us a path forward. We have diagnostics. We have vaccines. We have antivirals. What we need now is political will, equitable access, and durable public trust.
The future of Mpox won’t be shaped solely in high-tech labs or emergency briefings. It will be shaped in clinics, classrooms, crowded apartments, and community centers. It will be shaped by how well we listen, respond, educate, and invest — not just in science, but in people.
In that sense, Mpox may not just be a viral threat. It might be a test of our readiness — not just for the next virus, but for the world we want to build in its wake.