H5N9 Avian Influenza: A New Strain of Bird Flu
Introduction
So here we are again — another avian flu strain cropping up, this time H5N9. At first glance, it’s easy to think, okay, just another letter-number combo, and move on. But this one’s behaving just differently enough to make people pause.
The first confirmed cases turned up in poultry farms in Southeast Asia in early 2025. Nothing explosive, but the kind of thing that gets logged and flagged. Then a few more outbreaks — not large-scale, but scattered, persistent. Enough to suggest the virus wasn’t just a local anomaly. It was establishing itself.
This sort of emergence—quiet, regional, but brimming with potential—isn’t unique to flu. We’ve seen similar patterns in outbreaks like Mpox (Monkeypox), where animal-to-human transmission sparked global concern almost overnight. The mechanisms differ, but the early uncertainty and need for rapid surveillance are strikingly familiar.
The thing is, we’ve seen a lot of H5s before. H5N1, H5N8 — names that have already burned themselves into the public health memory bank. And H5N9 fits in that same clade. So naturally, everyone starts asking: Is it high-path? Can it jump species? What’s its receptor binding profile?
Initial genetic sequencing didn’t show anything catastrophic, but it did show something worth watching. The hemagglutinin looked closely related to other virulent strains, and the neuraminidase — the N9 — had a few markers that suggested it might be more mobile than average. Not alarming, not yet. But suspicious in the way early H7N9 once was.
What complicates it is the reassortment. This isn’t a virus that evolved in a straight line. It looks like it picked up gene segments from multiple parent strains, maybe in overlapping bird populations or along migratory routes. That always makes it harder to predict behavior. You don’t know whether it’ll settle into stable endemic circulation or take a sharp evolutionary turn.
So far, it’s sticking to birds. There haven’t been any confirmed human infections. But a few lab models are showing increased affinity for α-2,6 sialic acid receptors — the kind we have in our upper respiratory tract. That doesn’t mean human transmission is happening. But it means it’s possible, especially if it mutates further.
And from an industry standpoint, even if this never touches humans, it’s already a problem. Countries are starting to restrict poultry trade again. Some farms are culling preventively. Biosecurity is tightening. Because if there’s one thing the last two decades taught us, it’s that flu strains don’t respect borders — and they don’t wait for us to figure them out before moving.
So no, H5N9 isn’t a crisis. But it’s no footnote either. It’s early, it’s volatile, and it has potential. Which means it gets attention — not panic, but precision. And that’s exactly where we are now.
Virology and Pathogenesis
Let’s get into what this virus actually is. What makes H5N9 tick — genetically, structurally, and behaviorally?
So, first off: it’s an influenza A virus, which means it’s built with the usual eight-segment RNA genome. That’s typical for avian influenzas. But here’s where it gets interesting. The H5 refers to its hemagglutinin subtype — the protein that helps the virus attach to host cells. The N9 is the neuraminidase — the one that helps it break out of host cells and spread. Nothing new there in theory. But this combination? It’s rare.
Now, when we say “H5,” most people immediately think H5N1 — the high-path strain that’s caused deadly outbreaks in birds and humans since the early 2000s. H5N9’s hemagglutinin is closely related to that same lineage, which is why people perked up the moment it surfaced. You don’t need it to be identical — just close enough in the right domains — to start asking: How easily could this become high-path?
In terms of virulence, the current isolates suggest moderate pathogenicity in poultry, but there’s genetic plasticity in play here. That’s the real issue — plasticity. The virus has picked up gene segments through reassortment from different avian hosts. In other words, it’s stitched together from bits of other flu viruses that have circulated in waterfowl, domestic birds, and maybe even pigs.
This is where people in the field start worrying. Because when you’ve got reassortment happening in live bird markets, in areas with dense animal-human interfaces, the odds of spillover don’t just exist — they multiply.
And then there’s the receptor binding domain. That tiny region on the hemagglutinin determines whether the virus sticks to α-2,3-linked sialic acids (like in birds) or α-2,6-linked sialic acids (like in human airways). Right now, H5N9 still shows a strong preference for avian-type receptors. But — and it’s a big but — there are early indications of mutations (Q226L, G228S, if you’re watching the sequences) that might shift that binding affinity toward mammals.
So you start asking yourself: How much mutation does it take to cross that threshold? How many hosts does it need to pass through before one of them becomes a bridge species?
In terms of pathogenesis, if it behaves like its H5 cousins, you’d expect localized replication in the respiratory tract of birds, with potential for systemic spread in more susceptible species. It’s not causing mass die-offs yet — but you don’t need visible carnage to have a problem. Silent transmission is arguably more dangerous. It hides. It spreads before anyone reacts.
And if it gets into humans? That’s still theoretical — but the framework is there. Think about the past: H7N9 didn’t look dangerous at first either. Then it killed over 600 people. The virology doesn’t always scream; sometimes it whispers. Sometimes it mutates quietly in the corner of a wet market until it’s ready to move.
So here’s the big picture: H5N9 is a mosaic virus — genetically flexible, currently bird-focused, but not biologically locked down. It has the machinery. It’s experimenting, biologically speaking. Whether it figures out how to use what it has… that’s the part we don’t know yet.
Epidemiology
Where is H5N9 actually spreading? And more importantly — how is it moving?
Right now, the geographic footprint is still relatively narrow, but that’s changing. The earliest confirmed outbreaks came from poultry farms in Vietnam and southern China in early 2025. At first, it looked like a local issue — isolated farms, minimal export disruption, no human cases. But within a few months, detections started cropping up in northern Thailand, Cambodia, and even parts of eastern India.
And then it showed up in Poland.
That’s the moment people really started paying attention — when it moved into Europe. Because once a virus crosses continental lines, it stops being a local biosecurity issue and becomes a regional one — and potentially global.
So what’s driving the spread?
It’s not one thing. It never is. First, you’ve got wild migratory birds. These species are natural reservoirs for influenza A viruses, and they follow seasonal flyways that run from Southeast Asia through Siberia, down into the Middle East, and across to Eastern Europe. It’s entirely plausible that infected waterfowl are shedding the virus asymptomatically as they travel — and poultry farms near wetlands are vulnerable without even realizing it.

Then there’s live bird markets — high-density, low-regulation environments where viruses have a field day. These are the perfect mixing bowls for reassortment and amplification. One infected duck sold in a market can pass the virus to ten species in a day — and those birds are moved, sold, slaughtered, or released into the environment.
It’s a viral ecosystem that doesn’t just depend on birds—it’s about the spaces and species around them. If that sounds familiar, it’s because viruses like Oropouche Fever behave in surprisingly analogous ways, thriving in overlooked ecological corridors and spreading via vectors most people never consider until there’s a spike in human illness.
Let’s not forget poultry trade routes. Even countries with strict import standards have weak spots — informal shipments, poorly regulated distributors, or even legal imports from regions that aren’t reporting early cases because they haven’t seen them yet.
So transmission is happening through at least three channels:
- Wild bird migration (quiet, constant, and nearly impossible to control)
- Commercial and informal poultry trade (especially in border regions)
- Local spread between farms, often via contaminated equipment, feed, or worker movement
And here’s the part people sometimes miss: viruses like H5N9 don’t have to cause massive bird die-offs to move efficiently. Low-to-moderate pathogenic strains are harder to detect early, because they may not cause obvious clinical signs — especially in waterfowl, which can carry and shed the virus without showing illness.
So we start asking: Are we seeing the tip of the iceberg? Probably. Most surveillance systems rely on farmers reporting sick or dying birds. But if the birds don’t look sick — or if reporting leads to culling without compensation — cases go unreported. That means the true case map is likely wider than what’s on paper.
As for humans? No confirmed cases yet, but we’ve been here before. Early H7N9 outbreaks looked poultry-limited too. Then the spillovers started — sporadic, but deadly. That’s why monitoring is so aggressive right now, especially among poultry workers, live bird vendors, and veterinarians. Serosurveys are underway in high-risk populations in Vietnam and China, looking for silent seroconversion.
Which leads to the final question: How prepared are we to contain this if it escalates?
If H5N9 stays contained within poultry, we’re looking at an agricultural crisis — not a human one. But if human-adaptive mutations continue? Then it’s a very different story. And right now, the virus is outpacing our understanding of its movement. That’s not unusual. But it’s never comfortable.
Clinical Manifestations
So what does H5N9 actually do to the birds it infects? And what about people — have there been any signs it can cross over?
Starting with the birds: infections in poultry tend to present a mixed picture. In some flocks, especially chickens and turkeys, H5N9 has caused moderate to severe illness — respiratory distress, decreased egg production, swelling around the head, and sometimes sudden death. But unlike some earlier H5 viruses that caused catastrophic losses overnight, H5N9 can be a bit sneakier. Some birds show mild symptoms or even none at all, which makes early detection tricky.
Waterfowl, like ducks and geese, seem to carry the virus without getting very sick — that’s a classic pattern for many avian influenzas. These silent carriers become reservoirs, shedding the virus into the environment without any obvious warning signs.
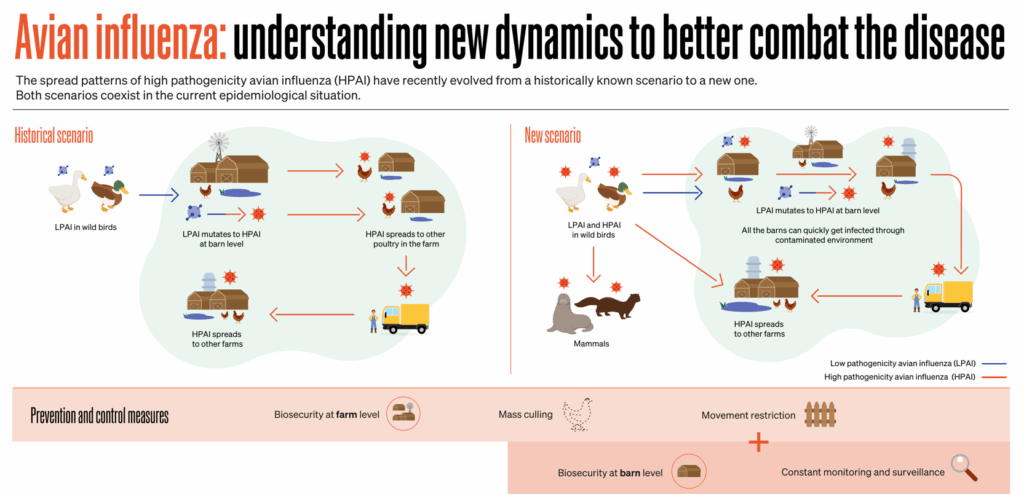
This asymptomatic shedding is a double-edged sword. It helps the virus spread quietly and persistently but complicates surveillance and control efforts. If you can’t tell who’s infected just by looking, how do you stop it?
What about humans? So far, confirmed human infections with H5N9 haven’t been documented. But that doesn’t mean zero risk. We’ve seen other avian influenzas, like H5N1 and H7N9, make the jump — sometimes with devastating effects.
If H5N9 were to infect a person, what might it look like? Based on what we know from related strains, initial symptoms would probably resemble severe flu: fever, cough, sore throat, muscle aches, sometimes progressing quickly to pneumonia or acute respiratory distress syndrome. In the worst cases, it can lead to multi-organ failure.
A big question hovering over H5N9 is how efficiently it could spread from person to person. Right now, the evidence suggests it’s limited to no human-to-human transmission. But influenza viruses are notorious for their ability to mutate, so that’s something researchers are watching closely.
Another unknown is whether H5N9 could cause more severe disease in certain groups — say, people with weakened immune systems, the elderly, or those with chronic conditions. That’s a question for ongoing clinical observation and research.
And of course, there’s always the risk of co-infection with seasonal flu or other respiratory viruses, which could potentially create a breeding ground for reassortment or increased virulence.
So to sum it up: in birds, the clinical picture is variable, from mild to severe, with silent carriers complicating detection. In humans, no cases yet, but the potential for severe disease exists if it crosses the species barrier — which is why vigilance is essential.
Diagnosis
So, how do you actually know if a bird — or potentially a person — has H5N9? It sounds straightforward, but in reality, diagnosis is a bit of a puzzle.
Starting with birds: the first clue usually comes from clinical signs — but as we just saw, those signs can be subtle or even absent. That means farmers or vets can’t rely solely on visible symptoms. When suspicious cases appear, samples are collected — typically swabs from the respiratory tract or cloaca, sometimes tissue samples if a bird has died.

These samples then go to specialized labs where molecular tests take center stage. The gold standard is RT-PCR (reverse transcription polymerase chain reaction), which looks for specific genetic sequences unique to H5N9. It’s fast and sensitive, but you need the right primers — which means labs must update their testing panels as new strains emerge.
One tricky part is that H5N9’s genome is reassorting — mixing gene segments with other influenza viruses. So sometimes a test that worked perfectly for last year’s flu might miss a new variant this year. That’s why sequencing the virus after detection is crucial. It helps track mutations, understand how the virus is evolving, and update diagnostic tools.
What about serology? Blood tests can detect antibodies showing prior exposure, which is useful in surveillance, especially in wild birds or humans exposed to poultry. But antibodies take time to develop, so serology isn’t great for early detection.
In humans, if someone shows flu-like symptoms and has a history of poultry exposure in an affected area, doctors will collect respiratory samples and send them for influenza typing. But here’s the catch: early symptoms overlap with many other respiratory infections. Without targeted testing, H5N9 infections could be missed or misclassified.
And let’s talk challenges.
Early diagnosis hinges on awareness. Farmers may hesitate to report sick birds because culling means economic loss. In some regions, surveillance systems are patchy, meaning viruses can circulate under the radar. Plus, lab capacity varies wildly — some places lack rapid PCR testing or sequencing, delaying identification.
So the diagnostic landscape is a mix of high-tech lab work and real-world hurdles. It’s not just about having the test; it’s about knowing when and where to use it — and making sure the results get communicated quickly to trigger response.
In short: diagnosing H5N9 is a race against time and evolution. The virus mutates, the symptoms hide, and surveillance gaps exist. But molecular tools, when deployed well, can catch it — giving us a fighting chance.
Treatment and Management
Alright, so imagine you’ve confirmed an H5N9 infection — in birds or, hypothetically, in humans. What do you do next? How do you treat it? And what are the challenges in managing this virus?
Starting with birds — the frontline of this battle — treatment options are limited. Unlike bacterial infections, influenza viruses don’t respond to antibiotics. So it’s mostly about containment: culling infected flocks, quarantining affected farms, and boosting biosecurity to stop spread.
There are antiviral drugs for birds, like amantadine or rimantadine, but their use is discouraged. Why? Because flu viruses develop resistance quickly, and widespread use can make the problem worse, not better. Plus, drug residues in food products raise safety concerns.
Vaccination in poultry is an option in some countries, but it’s tricky. Vaccines need to be well-matched to the circulating strain to be effective, and with H5N9’s reassortment and mutation, that’s a moving target. Also, vaccinated birds might still get infected and shed virus silently, complicating detection and control.
So prevention through strict biosecurity measures often takes precedence — controlling farm access, disinfecting equipment, monitoring bird health daily, and managing waste and water sources carefully.
Switching to humans — luckily, no confirmed H5N9 cases yet, but if it jumps, treatment protocols would likely mirror those used for other avian influenzas like H5N1 or H7N9. That means antiviral drugs such as oseltamivir (Tamiflu) or zanamivir would be frontline options. These drugs can reduce severity if given early but aren’t magic bullets. Resistance can develop, and availability may be limited in some regions.
Supportive care is crucial. Patients with severe flu symptoms often need hospitalization, oxygen therapy, and sometimes mechanical ventilation if respiratory failure develops. Managing complications like secondary bacterial infections is also a key part of care.
What about vaccines for humans? That’s a complicated topic. Because H5N9 is new, no approved vaccine exists yet. Vaccine development takes time — isolating the virus, creating candidate vaccine viruses, testing safety and efficacy, scaling production. It could be months, even years, before a vaccine is widely available if human infections begin to appear.
In the meantime, public health efforts focus on early detection, isolation of cases, and contact tracing to prevent spread. Stockpiling antivirals and developing updated vaccine platforms are part of pandemic preparedness strategies.
One big question is: can we stay ahead of the virus’s rapid mutation rate? That’s the core challenge. Antivirals can lose effectiveness, vaccines can become outdated, and containment efforts can be undermined by silent spreaders.
So management of H5N9 — whether in birds or humans — isn’t about a quick fix. It’s about constant vigilance, adaptability, and integrating veterinary and public health responses seamlessly.
Prevention and Control
So, if treatment options are limited and the virus keeps changing, what can actually be done to stop H5N9 in its tracks? How do we prevent this from turning into a bigger problem?
The answer starts on the farm — literally. Prevention in poultry is the cornerstone. Farms need robust biosecurity protocols. That means controlling access strictly: only essential personnel allowed, proper protective gear worn, and disinfection stations set up at every entry and exit point. It sounds simple, but in reality, it takes serious effort and resources.
Think about the daily routines that can inadvertently spread the virus — a worker moves from one barn to another without changing boots or clothes, equipment is shared between farms without thorough cleaning, feed or water sources get contaminated by wild birds. Each small slip can open a door for the virus.
Then there’s the challenge of live bird markets, especially in regions where they’re cultural staples and economic lifelines. These markets are viral hotbeds — crowded cages, multiple species mixed together, little regulation. Closing them outright often isn’t practical or even possible. So authorities try things like periodic market rest days for deep cleaning, better ventilation, and education campaigns to encourage safer practices.
And how about surveillance? You can’t stop what you don’t see. Regular testing of flocks, especially those near wetlands or migratory bird pathways, helps catch infections early. But again, that requires investment and cooperation from farmers, who might fear losing their livelihoods if outbreaks are reported.
On the human side, prevention means educating people who work closely with poultry — farmers, market workers, vets — about the risks and protective measures: wearing masks and gloves, practicing hand hygiene, avoiding direct contact with sick birds.
Public health agencies often run awareness campaigns during outbreaks to discourage risky behaviors, like handling or consuming sick or dead birds. But those messages have to balance cultural sensitivity and economic realities. Telling people not to sell or eat poultry without offering alternatives doesn’t always work.
Vaccination strategies in poultry can help reduce viral load and shedding, but as we discussed, matching vaccines to a rapidly evolving virus is tough. Still, some countries use targeted vaccination to contain outbreaks.
Finally, there’s the international dimension. Viruses don’t respect borders, so global cooperation is vital. Data sharing, coordinated surveillance, and joint response plans help catch new outbreaks early and prevent spread.
But even with all these measures, the virus is clever. Migratory birds don’t get biosecurity protocols. Illegal poultry trade happens. Human behaviors don’t change overnight.
So the real question is: how do we build systems that are flexible enough to adapt — that can respond quickly to new information and shifting risks?
Because stopping H5N9 — or any emerging avian flu — isn’t a one-time fix. It’s a continuous process, a balancing act between science, policy, culture, and economics.
Recent Developments (2025–2026)
So, what’s been happening with H5N9 lately? Has it done anything surprising or alarming in the past year or so?
The short answer is: yes, and no. The virus hasn’t exploded into a global crisis — yet. But it has made some noteworthy moves that keep experts on alert.
In 2025, several outbreaks were confirmed across Southeast Asia, as we talked about earlier — Vietnam, Thailand, Cambodia. These outbreaks were mostly contained through rapid culling and movement restrictions, but they showed that H5N9 was establishing itself in multiple avian populations simultaneously.
What really caught attention was the jump to Europe, particularly Poland, in late 2025. This wasn’t just a random isolated incident; genetic sequencing showed the virus there was closely related to the Asian strains, suggesting migratory birds or poultry trade routes played a role. It raised questions about how far the virus had already traveled undetected.
Meanwhile, surveillance efforts have ramped up dramatically. Labs across affected regions have improved their diagnostic capacity, using more rapid PCR testing and expanding sequencing efforts. This means new viral mutations are being spotted faster, giving a clearer picture of H5N9’s evolution.
Some of the mutations detected recently are in the hemagglutinin gene’s receptor-binding domain — exactly the places that determine how well the virus might infect humans. Although none have yet enabled efficient human-to-human transmission, these genetic tweaks underline the virus’s potential to adapt.
On the treatment front, research into antiviral effectiveness against H5N9 is ongoing. Some lab tests suggest oseltamivir still works well against current strains, but resistance mutations are possible, so scientists are monitoring carefully.
Vaccine development has also picked up pace. Candidate vaccines based on early H5N9 isolates are now in preclinical trials. That’s good news because it means if human infections begin, we won’t be starting from scratch. However, it’s too early to say when or if these vaccines will be needed or widely available.
There have also been some promising innovations in diagnostic tools — portable PCR units and rapid antigen tests designed for field use, helping vets and health workers get quick answers without waiting days for lab results.
So where does all this leave us? H5N9 remains a virus with real potential but without a crisis attached — a classic example of a threat on the horizon. That makes ongoing vigilance essential. Watching these genetic changes, tracking outbreaks, improving response speed — it’s all part of keeping ahead.
And the big question still looms: will this virus stay mostly an avian problem, or will it evolve in ways that push it into humans more consistently?
For now, we watch and learn.
FAQ: H5N9 Avian Influenza
What is H5N9?
H5N9 is a newly identified subtype of avian influenza A virus that has emerged primarily in poultry populations across Asia and parts of Europe since 2025. It is related to other H5 viruses known for affecting birds and occasionally humans.
Why is H5N9 important?
It poses a threat to the global poultry industry due to its pathogenicity in birds, which can cause economic losses. It also carries potential zoonotic risk—meaning it might infect humans if it mutates further.
How does H5N9 differ from other avian flu strains like H5N1?
While it shares similarities with other H5 strains, H5N9 has a unique genetic makeup resulting from reassortment, making its behavior and evolution less predictable.
Where has H5N9 been detected?
Initially found in Southeast Asia (Vietnam, Thailand, Cambodia), it has since been identified in parts of Eastern Europe, including Poland, likely spread by migratory birds and poultry trade.
What symptoms does H5N9 cause in birds and humans?
In birds, symptoms range from mild to severe respiratory distress and sometimes sudden death. No confirmed human cases exist yet, but if infection occurs, symptoms would likely resemble severe influenza.
How is H5N9 diagnosed?
Diagnosis relies on molecular techniques such as RT-PCR and sequencing from respiratory or cloacal samples. Early detection is challenging due to subtle symptoms and the virus’s genetic variability.
What treatment options exist for H5N9 infections?
In birds, treatment focuses on containment and biosecurity rather than antivirals. In humans, antiviral drugs like oseltamivir may be effective if administered early, though no specific treatments or vaccines are yet approved.
How can H5N9 spread be prevented?
Prevention involves strict biosecurity on farms, improved hygiene in live bird markets, surveillance of poultry and wild birds, public education, and international cooperation.
What recent developments have occurred with H5N9?
Surveillance and sequencing efforts have increased, revealing ongoing mutations that could affect infectivity. Preclinical vaccine trials are underway, but no human vaccines are available yet.
Final Thoughts
H5N9 isn’t a headline-grabbing pandemic right now — but it’s a reminder that influenza viruses are always evolving, always testing the boundaries between species. The fact that it’s popped up across continents and shows genetic flexibility means it deserves careful watching.
For now, it remains primarily a threat to birds and the poultry industry, but the potential human risk means that scientists and public health officials are staying alert, working to catch changes early, and preparing as best they can.
Understanding viruses like H5N9 is like watching a live experiment in evolution — it’s unpredictable, sometimes frustrating, but essential if we want to stay a step ahead. The best tools remain vigilance, collaboration, and rapid response.
Understanding viruses like H5N9 is like watching a live experiment in evolution — it’s unpredictable, sometimes frustrating, but essential if we want to stay a step ahead. And if we’ve learned anything from past crises like Ebola, it’s that our response is only as strong as the systems we’ve built in advance—surveillance, diagnostics, communication, and trust.
So stay informed, pay attention to credible updates, and trust that behind the scenes, experts are doing their best to keep this virus contained before it can do more harm.










