Candida Auris: A Multidrug-Resistant Fungal Pathogen
Introduction
When Candida auris was first described in 2009, it appeared as an obscure entry in the clinical microbiology literature — a yeast isolated from a patient’s ear canal in Japan. At the time, it drew little attention. Yet within just a few years, it emerged nearly simultaneously in hospitals on four different continents, each instance involving genetically distinct strains. That improbable pattern of emergence — without clear epidemiological links — immediately signaled that this was not a typical fungal pathogen.
Today, C. auris is recognized by global health authorities as a significant threat to public health. The Centers for Disease Control and Prevention (CDC) has classified it as an “urgent threat,” while the World Health Organization (WHO) lists it among its priority fungal pathogens. Its importance lies not only in its ability to cause invasive infections with high mortality, but also in its multidrug resistance, environmental persistence, and potential for causing large-scale outbreaks in healthcare facilities.
Unlike more familiar fungal infections such as those caused by Candida albicans, C. auris displays an unusual suite of characteristics: it can colonize the skin, resist treatment with multiple antifungal agents, survive on surfaces for weeks, and evade detection by conventional laboratory methods. Together, these traits make it especially difficult to control in hospital and long-term care environments — particularly in intensive care units and among immunocompromised or critically ill patients.
The rise of C. auris also reflects a broader shift in the landscape of infectious disease. Fungal pathogens, long overlooked in discussions of antimicrobial resistance, are now emerging as serious concerns in their own right. Increasing use of broad-spectrum antimicrobials, global travel, and climate change are all contributing to this shift. With C. auris, the medical community is facing one of the first clear examples of a fungal pathogen adapting rapidly to the modern healthcare ecosystem.
This article will explore Candida auris in depth — from its microbiological features and mechanisms of resistance, to its clinical presentation, patterns of transmission, and current challenges in diagnosis, treatment, and infection control. As the medical and scientific communities work to better understand and contain this emerging pathogen, it serves as a stark reminder of the dynamic and evolving nature of infectious threats.
Microbiology and Pathogenesis
Candida auris is a yeast-forming fungus that, under the microscope, appears unremarkable — yet genetically and clinically, it is anything but. Its biology underpins both its resistance to antifungal treatment and its ability to persist and spread in healthcare environments, making it a uniquely challenging pathogen.
Morphology and Growth Characteristics
C. auris typically grows as oval to elongate yeast cells, similar in size and appearance to other Candida species. However, it does not form pseudohyphae or germ tubes under standard laboratory conditions, which are typical features used to identify Candida albicans. This morphological simplicity can hinder rapid identification and contribute to misidentification in routine clinical microbiology labs, especially when relying on traditional phenotypic methods.
Growth is rapid under high-salt and high-temperature conditions, which may help explain its survival in a wide range of clinical environments. Colonies grow well at 37°C and even up to 42°C, indicating thermotolerance that is unusual for most fungal pathogens and may have facilitated its emergence in hospital settings and under global warming conditions.
Genetic Characteristics and Clades
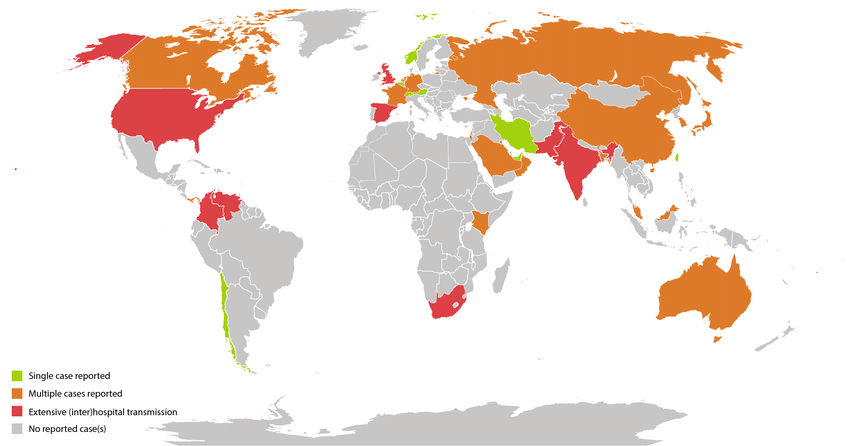
Whole-genome sequencing has revealed that C. auris comprises at least five major clades, each associated with distinct geographic regions: South Asia, East Asia, South Africa, South America, and most recently, Iran. These clades are genetically divergent, suggesting independent emergence events rather than spread from a single origin.
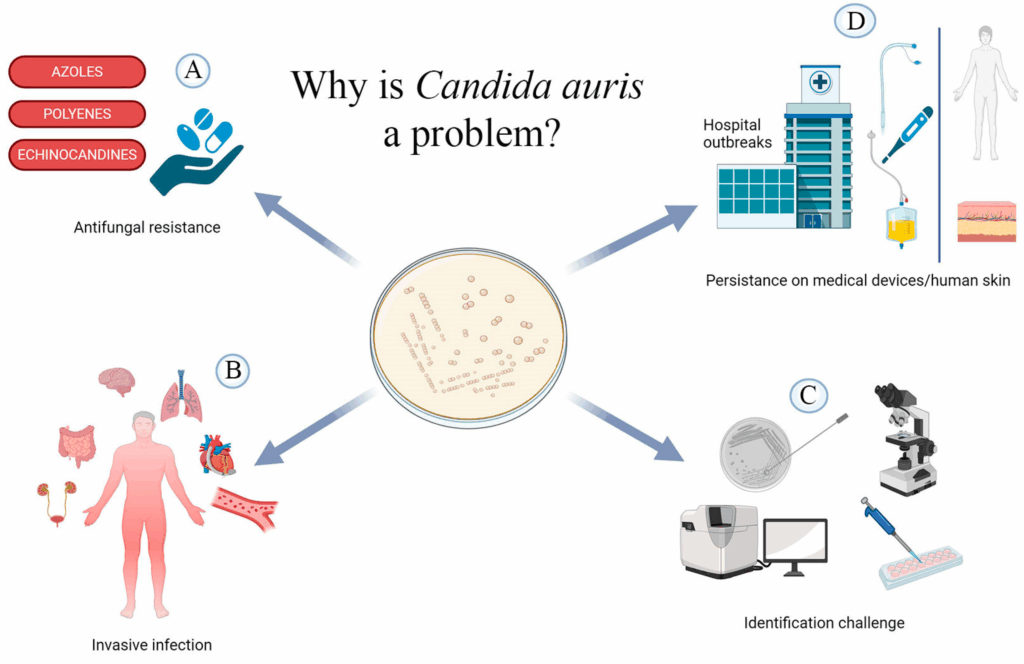
Despite their differences, all clades share several concerning features: multidrug resistance, environmental persistence, and potential for nosocomial transmission. The presence of genetic mutations in conserved regions such as the ERG11gene (linked to azole resistance) and FKS1 gene (linked to echinocandin resistance) underscores its capacity to survive first-line antifungal therapies.
Mechanisms of Resistance
Resistance in C. auris is primarily mediated by point mutations in key genes involved in the synthesis of ergosterol — a critical component of fungal cell membranes. Azole resistance is often conferred by mutations in ERG11, reducing drug binding and efficacy. Echinocandin resistance is less common but more worrisome, as it results from mutations in the FKS1 gene, which encodes the target of this antifungal class.
Multidrug resistance — defined as resistance to at least two antifungal classes — is found in a significant portion of isolates. Even more concerning, pan-resistance (resistance to azoles, echinocandins, and polyenes such as amphotericin B) has been documented in several countries, albeit still rarely. These pan-resistant strains leave clinicians with virtually no therapeutic options.
Biofilm Formation and Environmental Survival
C. auris exhibits a strong ability to form biofilms on both biological and inert surfaces. These biofilms, though not as robust as those of Candida albicans, still confer increased resistance to antifungal agents and disinfectants. This characteristic contributes to its persistence in healthcare environments and complicates efforts to eradicate it from surfaces and equipment.
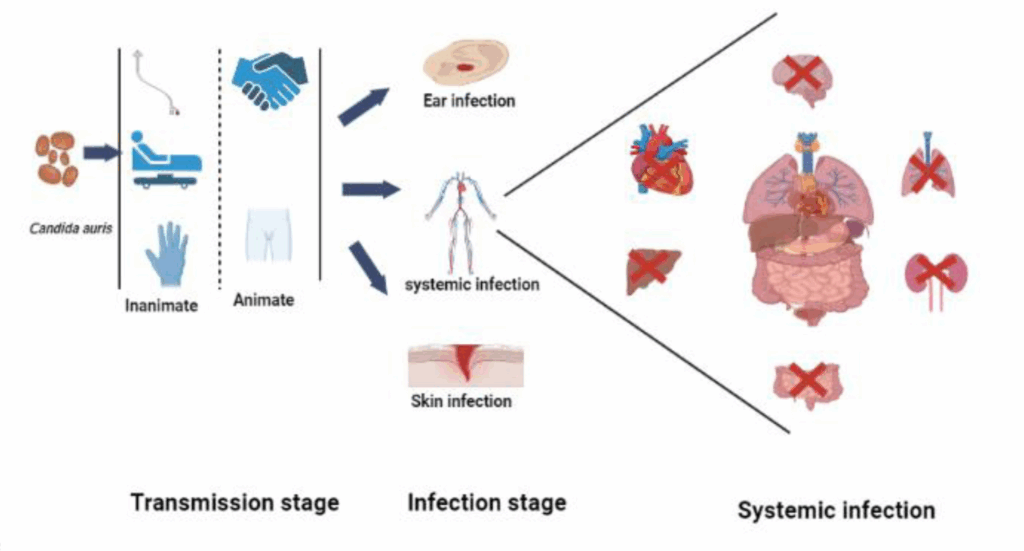
Moreover, its ability to colonize the skin — especially in moist areas like the axilla, groin, and nares — allows for silent transmission between patients and healthcare personnel. Colonized individuals may remain asymptomatic for extended periods, serving as reservoirs for future infections or outbreaks.
Pathogenesis
Invasion usually follows colonization, particularly in individuals with compromised skin or mucosal barriers. Once in the bloodstream, C. auris can cause candidemia and disseminated infections, with high associated morbidity and mortality. Unlike other fungal pathogens, C. auris infections often lack distinguishing clinical features, complicating timely diagnosis and delaying appropriate treatment.
Its tropism for the bloodstream and deep tissues, combined with diagnostic delays and therapeutic resistance, contributes to the high case fatality rate observed in outbreaks — often exceeding 30–40% in vulnerable populations.
Epidemiology
Since its first identification in 2009, Candida auris has transitioned from an isolated clinical curiosity to a globally recognized public health concern. Its emergence in multiple regions — including South Asia, South America, Africa, and East Asia — occurred almost simultaneously, with each region presenting genetically distinct clades. This unusual pattern has challenged conventional epidemiological explanations and prompted theories ranging from global antifungal pressure to environmental shifts related to climate change.
One of the most concerning aspects of C. auris is its persistence in healthcare settings. Unlike most other Candidaspecies, C. auris colonizes not only mucosal surfaces but also the skin and external environments, such as bedrails, doorknobs, and medical equipment. This environmental resilience contributes significantly to its transmission. Once introduced into a facility, it can rapidly spread among patients, particularly in high-dependency areas like ICUs.
The populations most affected include individuals with prolonged hospital stays, central venous catheters, tracheostomies, or recent broad-spectrum antimicrobial use. Immunocompromised patients are at highest risk for invasive infection, but colonization has been documented even in relatively healthy individuals under hospital care. Notably, asymptomatic carriage plays a critical role in outbreaks, making detection and containment much more difficult.
Geographically, the distribution of C. auris has followed the movement of patients, equipment, and healthcare staff. Nosocomial outbreaks have been reported in diverse settings — from tertiary hospitals in New York City to regional facilities in Colombia and the UK. These outbreaks often persist despite standard cleaning protocols, necessitating the use of more aggressive disinfectants and specialized surveillance strategies.
At the community level, C. auris has not demonstrated significant spread outside healthcare facilities. However, given its robust survival on skin and surfaces, there is concern that with continued evolution, it could eventually establish itself beyond hospital walls — particularly in long-term care environments or in the context of climate adaptation.
We covered related information on HKU5-CoV-2 in our article on zoonotic coronaviruses and cross-species spillover — a rising concern for fungal and viral pathogens alike.
In summary, the epidemiological profile of Candida auris is shaped by its environmental tenacity, ability to colonize without symptoms, and capacity to resist decontamination and antifungal treatment. These factors combine to make it a formidable challenge in both outbreak control and global surveillance.
Clinical Manifestations
The clinical presentation of Candida auris is notable less for any unique characteristics than for its ability to mimic more common infections, particularly in hospitalized and immunocompromised patients. Unlike Candida albicans, which often presents with mucocutaneous involvement or classic signs of candidiasis, C. auris tends to cause invasive infections that are often clinically indistinguishable from those caused by other pathogens — fungal or bacterial.
The most common manifestation is candidemia, typically occurring in patients with central venous catheters, recent surgeries, prolonged antibiotic use, or significant immunosuppression. Patients may present with persistent fever and sepsis-like features that do not respond to broad-spectrum antibacterial therapy. This nonspecific presentation delays appropriate diagnosis and treatment, particularly in institutions without access to rapid fungal diagnostics.
C. auris also causes deep-seated infections, including intra-abdominal, urinary tract, wound, and respiratory tract infections. However, isolating the organism from these sites does not always indicate true infection; colonization is common, and the distinction between colonization and invasive disease can be difficult to determine, especially in critically ill patients. In contrast to Candida albicans, which frequently colonizes the gastrointestinal tract, C. auris is more often found on the skin, including the axilla, groin, and nares — areas that can serve as reservoirs for spread and subsequent invasion.
The severity of disease caused by C. auris is compounded by the high prevalence of antifungal resistance and by its tendency to affect patients who are already critically ill. Mortality rates associated with invasive C. auris infections are substantial, ranging from 30% to over 60% in some cohorts, although these figures are difficult to interpret in isolation due to underlying comorbidities. Nonetheless, persistent fungemia and poor clinical response despite appropriate therapy are not uncommon, particularly in the context of multidrug- or pan-resistant strains.
Infection patterns also vary depending on patient population and clinical setting. In neonatal intensive care units, outbreaks have involved cases of bloodstream infections in preterm infants, where signs are subtle and often overlap with bacterial sepsis. In adult populations, invasive disease is frequently associated with mechanical ventilation, renal replacement therapy, and long-term hospitalization, especially in units with high device utilization.
In summary, Candida auris does not announce itself with distinct clinical hallmarks. Its danger lies in its diagnostic ambiguity, its resistance to treatment, and its ability to exploit vulnerable patients in high-risk settings. Effective clinical suspicion relies heavily on contextual factors — recent hospitalization, unexplained fever, failure of antibiotics — rather than on specific signs or symptoms. This underscores the importance of rapid laboratory diagnosis and close coordination between clinical and microbiological teams.
Diagnosis
Diagnosing Candida auris is not as straightforward as identifying many other fungal pathogens. While the organism itself is not particularly rare in modern clinical microbiology labs anymore, recognizing it correctly — and quickly — remains a major challenge.
In many institutions, routine yeast identification methods are not designed to detect C. auris accurately. This is particularly true of conventional biochemical platforms like API 20C AUX, VITEK 2 YST, or older versions of BD Phoenix and MicroScan. These systems tend to misidentify C. auris as a range of other yeasts — most commonly Candida haemulonii, but also Candida famata, Rhodotorula glutinis, or even Saccharomyces cerevisiae. These misidentifications can delay the appropriate response, especially during an outbreak when early recognition is crucial.
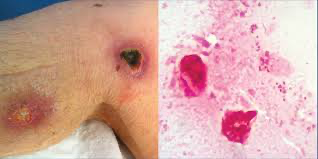
So what works? Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) has become a key diagnostic tool — but only if the reference database is up to date. Some early MALDI systems failed to include C. auris spectra, leading to false negatives or incorrect species-level assignment. Fortunately, as awareness of C. auris has increased, more laboratories have begun using enhanced libraries that include multiple clades of C. auris, improving both speed and accuracy of identification.
Molecular techniques offer another reliable approach. PCR-based assays targeting species-specific DNA regions can confirm C. auris rapidly from both cultures and clinical samples. However, these tests are not yet widespread in many clinical microbiology labs and are more likely to be found in national reference centers or during outbreak investigations. Whole-genome sequencing, while primarily used for surveillance and clade assignment, also plays a growing role in epidemiologic tracking, especially during multi-facility outbreaks.
Another diagnostic wrinkle lies in colonization screening. Because C. auris often colonizes the skin and mucosa without causing immediate infection, active surveillance is essential in outbreak-prone environments. Swabs from the axilla, groin, and nares are commonly used for this purpose. Culture-based methods work but are slow; PCR-based colonization screening has helped reduce turnaround time, allowing for faster implementation of infection control measures.
Antifungal susceptibility testing is a critical companion to species identification. While commercial broth microdilution systems provide MIC data, interpreting these values is complicated by the absence of universally accepted clinical breakpoints for C. auris. The CDC has published tentative interpretive criteria, but clinicians still face uncertainty, especially with isolates that fall into the “intermediate” or “elevated MIC” range. Resistance to fluconazole is common; echinocandin resistance is less frequent but far more problematic when encountered.
One of the most dangerous consequences of delayed or inaccurate diagnosis is inappropriate therapy. Empiric antifungal regimens that work well for Candida albicans or glabrata may have limited effect against C. auris, especially if the isolate turns out to be multidrug- or pan-resistant. The window for effective treatment is often narrow in critically ill patients, which makes diagnostic speed as important as accuracy.
Ultimately, the diagnosis of C. auris infections requires both technological capacity and clinical vigilance. Institutions that rely solely on conventional methods may miss early cases — or misinterpret colonization as contamination. Laboratories must not only have the right tools, but also the awareness and protocols to use them proactively when C. auris is suspected or confirmed in a patient or unit.
Treatment and Management
Treating Candida auris infections presents a complex clinical challenge that stems largely from its frequent resistance to multiple antifungal drugs and its ability to persist in difficult-to-eradicate reservoirs within patients. Unlike many fungal infections where standard therapy with fluconazole or echinocandins reliably succeeds, C. auris forces clinicians to carefully reconsider both drug choice and duration of therapy.
The first-line treatment recommended by most guidelines is an echinocandin — drugs like caspofungin, micafungin, or anidulafungin. These agents inhibit fungal cell wall synthesis and remain effective against the majority of C. aurisisolates. But what happens when resistance to echinocandins is encountered? Though less common than azole resistance, echinocandin resistance is increasingly reported, particularly among strains from certain geographic clades. This resistance often correlates with mutations in the FKS1 gene, leading to treatment failure and poorer outcomes.
When echinocandin resistance is confirmed or strongly suspected, amphotericin B becomes the next line of defense. Amphotericin B, a polyene antifungal, binds to ergosterol in fungal membranes, causing cell death. However, amphotericin B therapy is associated with significant nephrotoxicity and infusion-related side effects, which can limit its use, especially in critically ill or renal-impaired patients.
The widespread resistance to azoles — especially fluconazole — severely limits their role in treatment. Fluconazole resistance is reported in up to 90% of C. auris isolates globally, and this resistance mechanism is often stable and hard to overcome.
Given these limitations, combination therapy is sometimes considered, although robust clinical trial data are lacking. Combinations like echinocandin plus amphotericin B or echinocandin plus azole have been used in salvage therapy or persistent infections, but the evidence is largely anecdotal and based on case reports or small series.
Another key aspect of management involves removal or replacement of indwelling devices such as central venous catheters, which frequently serve as portals for bloodstream invasion. Without addressing these reservoirs, antifungal therapy alone may be insufficient to clear infection.
Treatment duration is not standardized but generally mirrors protocols for other invasive candidiasis — typically at least two weeks after documented clearance of bloodstream infection and resolution of symptoms. However, persistent colonization and difficulty in sterilizing body sites can prolong therapy or necessitate repeated courses.
Because of its capacity to colonize skin and survive on surfaces, C. auris infections require a comprehensive infection control approach alongside medical treatment. Failure to control environmental reservoirs can lead to reinfection or new outbreaks, complicating patient management.
What about new or investigational treatments? Research is ongoing into novel antifungal agents with activity against resistant C. auris, including the tetrazoles and echinocandin-like drugs with different binding profiles. Additionally, adjunctive therapies such as antifungal lock therapy for catheters or immunomodulatory approaches are under investigation but remain experimental.
In summary, managing Candida auris infections demands not only careful antifungal selection tailored to susceptibility profiles but also aggressive source control and environmental interventions. The therapeutic landscape remains challenging, highlighting the urgent need for new drugs and clinical trials focused specifically on this emerging pathogen.
Prevention and Control
Preventing Candida auris transmission in healthcare settings is one of the most critical challenges clinicians and infection control teams face today. Unlike many pathogens, C. auris combines a remarkable ability to survive on skin and environmental surfaces with widespread multidrug resistance — making traditional infection control measures sometimes insufficient.
Part of the reason C. auris is so hard to control lies in its persistence. This fungus can survive on surfaces such as bed rails, chairs, and medical equipment for weeks, even after routine cleaning. Standard disinfectants commonly used in hospitals, like quaternary ammonium compounds, often have limited efficacy against C. auris. This means that environmental reservoirs can serve as continuous sources of transmission, particularly in high-risk areas such as intensive care units.
Current recommendations emphasize enhanced environmental cleaning using agents with proven fungicidal activity, such as chlorine-based disinfectants or hydrogen peroxide vapor systems. Frequent and thorough cleaning of patient rooms, equipment, and common areas is essential.
Hand hygiene remains foundational. Since C. auris colonizes the skin, especially in moist areas, healthcare workers’ hands can easily become vectors. Alcohol-based hand rubs are effective against C. auris, but compliance with hand hygiene protocols must be rigorously enforced.
Isolation precautions are also crucial. Patients known or suspected to be colonized or infected with C. auris should be placed in single rooms with contact precautions — including the use of gowns and gloves by healthcare personnel. However, given the difficulty in rapidly identifying colonization, proactive screening of high-risk patients, such as those transferred from other facilities with known outbreaks, is recommended.
Screening protocols often involve swabbing axilla, groin, and nares to detect colonization. When colonized patients are identified, cohorting and dedicated staffing can help prevent cross-transmission.
Unfortunately, controlling C. auris outbreaks has proven notoriously difficult. Several documented outbreaks have persisted for months or even years despite aggressive interventions. This persistence underscores the importance of early detection, rigorous infection control training, and multidisciplinary coordination involving microbiology, clinical teams, environmental services, and hospital leadership.
While C. auris is primarily a healthcare-associated pathogen, its presence in long-term care facilities and nursing homes has increased. These settings pose additional challenges because residents often have prolonged stays, multiple comorbidities, and frequent hospital transfers — all risk factors for colonization and infection. Infection control practices in these environments may be less stringent or inconsistently applied, facilitating spread.
Efforts to extend surveillance, education, and infection prevention beyond acute care settings are therefore crucial to curbing community reservoirs.
You may also be interested in our detailed exploration of the Machupo virus — another under-recognized but highly dangerous pathogen that thrives in healthcare-challenged settings.
In summary, Candida auris prevention hinges on a combination of vigilant surveillance, stringent environmental cleaning with effective disinfectants, strict hand hygiene, patient isolation, and active communication across healthcare networks. While progress has been made, the stubborn resilience of this pathogen demands ongoing investment in infection control infrastructure and innovation.
Recent Developments (2025–2026)
In the past two years, Candida auris has continued to challenge healthcare systems worldwide, both through persistent outbreaks and the emergence of strains resistant to all major antifungal drug classes. These pan-resistant isolates have been reported from multiple continents, underscoring the increasing difficulty clinicians face when managing invasive infections. The spread of such strains highlights the urgent need for novel therapeutic options and reinforces the critical importance of antifungal stewardship to slow resistance development.
Diagnostic capabilities have advanced notably. Laboratories have integrated rapid molecular assays, allowing identification of C. auris directly from blood samples and surveillance swabs within hours rather than days. This accelerated detection is crucial for timely infection control interventions. Furthermore, the application of whole-genome sequencing during outbreak investigations has provided unprecedented insight into transmission dynamics, revealing complex patterns of spread between healthcare facilities and geographic regions. These genomic tools have become central to outbreak management and public health surveillance.
Therapeutic developments have also progressed. New antifungal agents, including members of the tetrazole class such as opelconazole and oteseconazole, show promising in vitro activity against multidrug-resistant C. auris strains. Clinical trials are underway to determine their efficacy and safety profiles. In parallel, combination antifungal therapies and optimized dosing regimens informed by pharmacokinetic and pharmacodynamic data are being explored to improve patient outcomes and mitigate resistance.
Infection prevention strategies have evolved to incorporate advanced environmental disinfection technologies. Ultraviolet-C (UV-C) light and hydrogen peroxide vapor systems are increasingly employed as adjuncts to manual cleaning, demonstrating enhanced effectiveness in reducing environmental contamination during outbreaks. The importance of coordinated regional surveillance and data sharing among healthcare institutions has become clearer, facilitating early detection and synchronized responses that limit transmission.
Emerging concerns include the potential influence of climate change on the epidemiology of C. auris and other fungal pathogens. Rising global temperatures may contribute to adaptation and environmental persistence, although the full extent of this effect remains under investigation. Sporadic environmental isolations of C. auris outside healthcare settings suggest that vigilance is warranted to monitor possible community establishment.
Overall, recent developments in diagnostics, therapeutics, and infection control represent important steps forward. However, Candida auris remains a formidable pathogen, demanding ongoing research, multidisciplinary collaboration, and sustained public health efforts to mitigate its impact.
Frequently Asked Questions (FAQ)
What is Candida auris and why is it important?
C. auris is a multidrug-resistant fungal pathogen that has emerged globally as a significant cause of invasive infections, particularly in healthcare settings. Its importance lies in its resistance to multiple antifungal agents, ability to persist on surfaces, and capacity to cause difficult-to-control outbreaks.
How does Candida auris differ from other Candida species?
Unlike other Candida species, C. auris is notable for its high level of antifungal resistance, persistence on skin and environmental surfaces, rapid emergence across multiple continents, and difficulty in accurate laboratory identification.
Who is at risk for Candida auris infection?
Patients with prolonged hospital stays, especially those in intensive care units; those with central venous catheters, mechanical ventilation, or recent broad-spectrum antibiotic use; and immunocompromised individuals are at increased risk.
What are the typical clinical manifestations?
Most commonly, C. auris causes bloodstream infections (candidemia) and invasive disease in critically ill patients. Symptoms are nonspecific and often resemble other causes of sepsis or fungal infections.
How is Candida auris diagnosed?
Accurate diagnosis requires advanced laboratory techniques such as MALDI-TOF mass spectrometry with updated databases or molecular methods like PCR. Conventional biochemical tests often misidentify it.
What treatment options are available?
Echinocandins are first-line therapy, but resistance is increasing. Amphotericin B remains a salvage option despite toxicity concerns. New antifungal agents and combination therapies are under investigation.
How can transmission be prevented?
Effective prevention requires rigorous infection control measures including strict hand hygiene, patient isolation, enhanced environmental cleaning with fungicidal disinfectants, active surveillance, and coordinated outbreak management.
What are the recent advances in managing Candida auris?
Rapid molecular diagnostics, whole-genome sequencing for epidemiologic tracking, new antifungal agents in clinical trials, and advanced environmental disinfection technologies have improved detection and control.
Final Thoughts
Candida auris represents a paradigmatic example of an emerging multidrug-resistant fungal pathogen that exploits modern healthcare environments. Its rapid global spread, diagnostic challenges, and limited treatment options underscore the need for vigilance, innovation, and collaboration across clinical, laboratory, and public health domains.
As awareness and research efforts intensify, improvements in diagnostic speed and accuracy, antifungal therapeutics, and infection prevention offer hope for better control. However, the persistent resilience of C. auris demands sustained attention and investment to prevent further morbidity and mortality from this formidable pathogen.









