Machupo Virus: Bolivian Hemorrhagic Fever
Introduction
Have you ever heard of the Machupo virus? If you haven’t, you’re not alone — and if you have, you might associate it with something out of a medical thriller. It’s real, though. Deadly real. And it changed the course of public health in Bolivia.
So, what exactly is the Machupo virus? It belongs to the Arenavirus family, a group of viruses that often live quietly in nature but can cause severe disease when they cross into humans. Machupo is one of those viruses overshadowed by more notorious relatives like Ebola or Marburg, yet it’s no less serious. It causes Bolivian Hemorrhagic Fever (BHF), a disease with a name as ominous as its effects — high fever, internal bleeding, and a disturbingly high risk of death.
Why did it emerge in Bolivia in the 1950s? The first recognized outbreak occurred in San Joaquín, a remote area in northeastern Bolivia. At the time, doctors were baffled by the strange and rapidly fatal illness striking people. Patients were dying quickly with symptoms like severe bleeding, shock, and neurological changes — all terrifying signs of something deadly and poorly understood. It wasn’t until the early 1960s that researchers identified the Machupo virus and began piecing the puzzle together.
What does “Machupo” even mean? The virus is named after the Machupo River in Bolivia, near where the initial cases appeared. Since then, it has come to represent more than a local outbreak; it serves as a case study in how environmental change, human movement, and zoonotic diseases collide — often with devastating consequences.
Why are we still talking about Machupo in 2025? Because this virus hasn’t disappeared. Like many arenaviruses, Machupo quietly persists in rodent hosts, waiting to reemerge when conditions are right. And with climate change, deforestation, and growing global mobility, those conditions are becoming more frequent, not less.
Machupo virus is not just a piece of Bolivian history; it’s part of a broader, urgent conversation about emerging infectious diseases. How prepared are we? How can we contain outbreaks before they spread beyond borders? And what can past experience teach us about protecting the future?
Virology and Pathogenesis
To understand what makes Machupo so dangerous, we have to zoom in — way down to the molecular level. Machupo is an RNA virus with a bisegmented, ambisense genome. In simpler terms, its genome is split into two RNA strands, each encoding proteins both ways, making it highly efficient and tricky to target.
One segment codes for proteins essential for viral replication, like the nucleoprotein and polymerase enzyme, while the other codes for the glycoprotein complex — the spikes on the virus’s surface that act as biochemical lockpicks, allowing it to latch onto and enter human cells.
Infection begins when aerosolized particles from infected rodent urine or feces are inhaled, delivering the virus to the respiratory tract. The glycoproteins bind to receptors on host cells — mainly alpha-dystroglycan — and the virus is engulfed through endocytosis. Inside the cell, Machupo unpacks its RNA and replicates aggressively, hijacking the cell’s machinery to produce thousands of new viruses. These then burst out, spreading to neighboring cells, causing widespread tissue damage — especially to the vascular system, which explains the severe bleeding symptoms.
But Machupo’s real menace lies in its stealth. It doesn’t just evade the immune system; it actively suppresses it by inhibiting interferon production, which normally signals the body’s alarm. This early silencing gives the virus a head start, allowing it to multiply rapidly. By the time the immune system responds, it often overreacts with a cytokine storm — a flood of immune signals that cause more harm than good, leading to vascular leakage, bleeding, and organ failure.
In a way, Machupo is a viral mastermind. It kills not with brute force but by subtle evasion and manipulation, turning the body’s defenses against itself — and that’s what makes it so terrifying.
Epidemiology
Where does Machupo live when it’s not infecting people? The virus nests deep in nature, circulating quietly in its main reservoir, the rodent Calomys callosus — the large vesper mouse. This species has evolved with the virus and remains unaffected by infection, making it an efficient carrier. The virus replicates in the mouse’s salivary glands, urine, and feces, contaminating the environment. Humans typically become infected through contact with contaminated dust, food, or surfaces, often in rural Bolivian communities where houses are close to the ground and rodent control is limited.
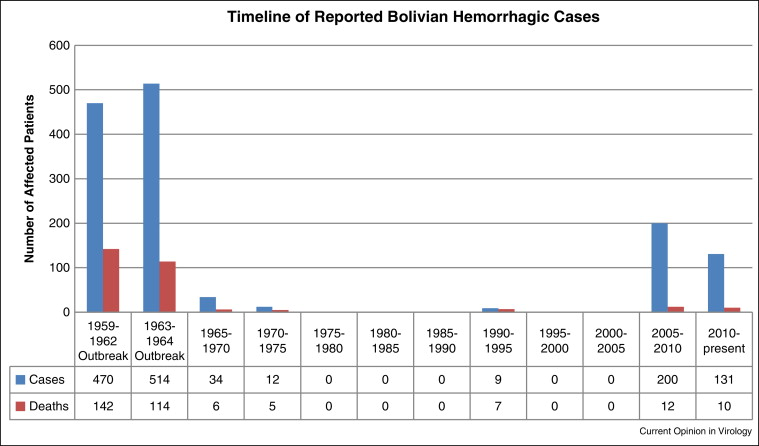
Machupo has so far only been found in Bolivia, particularly the Beni Department in the Amazon basin, but ecological boundaries aren’t fixed. Rodents move, people move, and climate and land use changes could allow similar viruses or Machupo itself to spread into neighboring regions.
Outbreaks are sporadic, influenced by rodent population booms after environmental changes, human activities like deforestation, and seasonal factors such as rainy seasons that aerosolize dried rodent waste.
Who is most vulnerable? While the virus can infect anyone, rural agricultural workers, children, and those living in poor housing conditions are especially at risk. These are not only medical but also social and economic risk factors, highlighting the intersection of disease emergence with inequality and infrastructure.
Human-to-human transmission is possible but rare, mostly occurring through contact with infected bodily fluids like blood, saliva, or vomit. This puts healthcare workers and family caregivers in high-risk groups, especially where protective equipment is limited.
The way diseases like Machupo emerge isn’t just about virology—it’s about ecology, infrastructure, and the choices we make about land use and housing. Our article on the Avian Influenza H5N, similarly explores how environmental disruptions can spark outbreaks in ways that often catch public health systems off guard.
Machupo virus sits at the intersection of environment, human behavior, and viral biology. It’s not just about the mouse — it’s about how we share space with it, and how ready we are when that balance tips.
Clinical Manifestations
Bolivian Hemorrhagic Fever often starts subtly — like a cold or flu. Mild fever, headache, muscle pain — symptoms that mask its deadly potential. The incubation period is usually 7 to 14 days, after which symptoms rapidly worsen: high fever, eye pain, nausea, and neurological symptoms such as confusion or seizures.
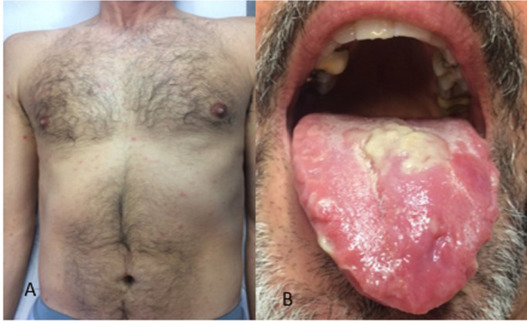
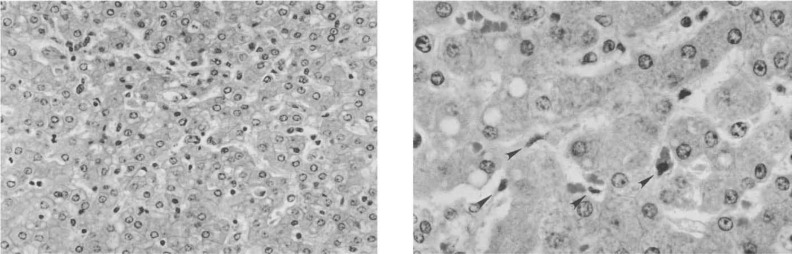
Bleeding — the hallmark of the disease — may begin with gums or nose and progress to internal hemorrhaging visible in vomit or stool. The skin bruises easily, and small purple spots may appear. This internal unraveling results from damage to endothelial cells lining blood vessels and a massive inflammatory response that compromises vascular integrity. Blood vessels leak, clotting factors are depleted, and the immune system’s cytokine storm leads to tissue damage, organ failure, and shock.
Fatality rates range from 20% to 30%, higher where medical care is limited. Survivors often face a prolonged recovery with neurological symptoms.
Machupo is no ordinary tropical fever. It overwhelms systems quickly, demanding rapid recognition and intervention.
Diagnosis
Diagnosing Machupo virus isn’t like flipping a switch. It’s more like fumbling for a light in a dark, unfamiliar room — one where time is running out, and the consequences of a wrong guess are measured in lives.
Most patients don’t show up saying, “I think I have Bolivian hemorrhagic fever.” They arrive with a fever, headache, maybe some nausea — symptoms that are so general, they could be anything from a seasonal virus to one of the many endemic tropical diseases that circulate in Bolivia. In fact, unless you’re in the middle of a known outbreak, most clinicians won’t suspect Machupo at all — at least not at first.
This is where the challenge begins. In the early stages, Machupo virus masquerades as other illnesses — dengue, leptospirosis, even typhoid. All of these can start with fever, malaise, body aches. And in resource-limited settings, it’s not always easy — or possible — to order a battery of tests. So, the initial diagnosis is often based on clinical suspicion and context: Is the patient from a known endemic area? Have there been recent reports of hemorrhagic cases nearby? Have family members been sick or died recently? These clues start to shape the story.
But suspicion isn’t enough. You need confirmation — and that’s where things get more technical.
The gold standard for detecting Machupo virus is reverse transcription polymerase chain reaction (RT-PCR), a method that amplifies viral RNA from a blood sample. If it’s there, the test will find it — assuming you have the right equipment, trained personnel, and a sample taken early enough in the disease course. That’s a lot of “ifs” in the remote corners of Bolivia, where Machupo tends to emerge.
Another method, ELISA (enzyme-linked immunosorbent assay), can detect antibodies or viral antigens. Antigen tests can catch the virus in the acute phase, while IgM or IgG antibody tests might suggest more about the timeline — whether the infection is recent or if someone has recovered. But again, these tests are not always readily available in frontline clinics. They often require samples to be sent to regional or national labs, delaying results.
And delay can be fatal.
That’s the cruel twist: by the time a test confirms Machupo virus, the patient may already be deep in the critical phase — bleeding, seizing, or in shock. The window for effective supportive care is small, and the virus doesn’t wait for paperwork.
In these moments, healthcare isn’t just clinical—it’s logistical. The challenge isn’t knowing what to do, but whether the system in place can actually do it.
You may also be interested in our detailed exploration of “Ascites Treatment in 2025”, which connects closely with this topic. That article looks at how diagnostic delays and infrastructure gaps affect outcomes in resource-limited settings—an issue just as urgent with Machupo.
There’s also a safety angle here. Working with a live Machupo virus requires biosafety level 4 (BSL-4) containment — the highest level of biocontainment we have. That’s reserved for viruses with no widely available vaccine or treatment, and where aerosol transmission is a risk. So, while samples can be tested for genetic or antigenic signatures in BSL-3 labs under strict conditions, actual virus isolation — growing it in cell culture — is a rare and risky endeavor.
Clinicians also rely on basic lab indicators to hint at something serious, even if they can’t name it right away: low platelet counts, elevated liver enzymes, signs of kidney injury, abnormal clotting profiles. These findings don’t scream “Machupo,” but they whisper that something is seriously wrong.
So diagnosis, in practice, is layered. It’s a mix of pattern recognition, contextual knowledge, and lab support — when it’s available. In the best-case scenario, suspicion leads to rapid isolation and supportive care. In the worst case, the diagnosis arrives as a postmortem formality.
And maybe that’s the hardest part. With diseases like Machupo, where the outbreak areas are remote and medical resources are thin, diagnosis isn’t just a medical task — it’s an act of urgency, improvisation, and often, of gut instinct.
Treatment and Management
When someone is diagnosed with Bolivian Hemorrhagic Fever — or even when the suspicion is high enough — there’s an immediate, uncomfortable realization: we don’t have a cure.
There’s no antiviral drug approved specifically for the Machupo virus. No widely available vaccine. No magic bullet. And so, the response defaults to the fundamentals — supportive care, intensive monitoring, and, if possible, aggressive early intervention to stabilize the patient while their body fights off the infection.
But what does “supportive care” really mean in this context?
It’s not a throwaway term. In the case of Machupo, supportive care is a matter of life and death. Patients need fluids — often intravenously, and in carefully measured doses. They need electrolyte correction, oxygen therapy, monitoring for bleeding, and sometimes transfusions when blood loss becomes critical. In more severe cases, they may require vasopressors to maintain blood pressure, or dialysis if kidney failure sets in.
That kind of care demands infrastructure: skilled staff, reliable power, access to medications, laboratory support — not always a given in the areas where Machupo virus strikes.
And then there’s the challenge of timing. The virus moves fast. By the time a patient is admitted with severe symptoms, the immune system may already be spiraling out of control. This is when we see cytokine storms, hemorrhage, neurological symptoms — and where treatment becomes more about damage control than true reversal.
Now, there is one glimmer of hope in the form of ribavirin, an antiviral that has shown some efficacy against other arenaviruses, like Lassa virus. But with Machupo, the evidence is limited. Animal studies have shown promise, and some compassionate-use cases suggest it might help — if given early, ideally within the first few days of illness. But here’s the problem: early diagnosis is rare, and ribavirin isn’t always stocked or available in the regions that need it most. So the drug exists, but access and timing remain serious barriers.
Meanwhile, experimental treatments are being explored. Some researchers are looking into monoclonal antibodies, modeled after the success seen with Ebola and other hemorrhagic viruses. These would be lab-engineered antibodies that bind to the virus and block it from infecting cells. Promising in theory. But still largely untested in humans, and definitely not something you’ll find in a rural Bolivian clinic today.
And what about vaccines?
There was a Machupo virus vaccine candidate developed decades ago, originally using an attenuated strain. It even showed protective effects in non-human primates. But for various reasons — safety concerns, funding gaps, low commercial interest — it never made it past the experimental stage. As of now, no vaccine is available to the public. Some research institutions have restarted vaccine efforts using newer platforms, including mRNA and viral vector technology. But these are in early phases, and there’s no rollout on the immediate horizon.
So, what can we realistically do?
First and foremost, we manage the environment: isolate patients, protect healthcare workers, prevent further transmission. Personal protective equipment (PPE), proper waste handling, limited contact with bodily fluids — these are critical. In outbreak settings, infection control becomes as important as direct patient care.
The rest of it — antivirals, vaccines, cutting-edge therapies — feels just out of reach. Not because we lack the science, but because we haven’t invested deeply enough in bringing that science to the people who need it most.
And so for now, treatment of Machupo virus remains a balance between medical resilience and resource limitations. Between what we know, and what we can actually do. Between the life-saving potential of modern medicine — and the harsh realities on the ground.
Prevention and Control
If there’s one lesson Machupo virus keeps teaching us — over and over — it’s this: it’s far easier to prevent an outbreak than to respond to one. Once people start bleeding, once fear takes hold, once the health system is strained — you’re already on the defensive.
But prevention? That’s where strategy lives. That’s where quiet, unglamorous, long-term work can save hundreds of lives.
So where does prevention actually begin?
Not with vaccines, not yet — though that would change everything. It begins, believe it or not, with rodents. Specifically, the Calomys callosus mouse, which is both the virus’s home and its taxi. You stop the mouse, you slow the virus.
But here’s the catch: this isn’t a villainous lab rat you can just exterminate. C. callosus is native to the region, highly adaptive, and thrives in exactly the kinds of environments where people are most vulnerable — poor housing, open food storage, stacked firewood, thatch roofs, cracked walls. In other words, this is not just a rodent problem — it’s a poverty problem.
Efforts to reduce exposure to Machupo virus focus heavily on rodent control and environmental hygiene. That means:
- Improving housing conditions — sealing cracks, raising floors, adding barriers between indoor spaces and rodent-infested zones.
- Proper storage of grains and food supplies — often using rodent-proof containers or elevated platforms.
- Clearing vegetation and debris around homes where rodents nest.
- Community education programs — teaching people to recognize rodent droppings, safely clean contaminated areas, and reduce contact with rodents and their waste.
Simple on paper, right? But the real world complicates things. These recommendations require materials, labor, time, and local trust. They often depend on NGOs or health ministries coordinating with villages, tailoring messaging to culture, even redesigning homes — not just handing out pamphlets.
And then there’s outbreak response.
When a suspected case of Bolivian hemorrhagic fever appears, the priority shifts fast. Public health teams mobilize to identify and isolate cases, trace contacts, and set up quarantine zones if needed. PPE becomes critical. So does public messaging — because panic spreads faster than the virus. People need to know what’s happening, how to stay safe, and what symptoms to watch for, without resorting to fear or superstition.
Healthcare workers, by the way, are at particular risk. Machupo virus can spread via direct contact with bodily fluids — and in under-resourced clinics, gloves, gowns, and face shields aren’t always guaranteed. Part of prevention, then, is ensuring that health systems are supplied and trained before outbreaks begin, not in response to them.
Internationally, there’s been talk for years about including Machupo in priority pathogen watchlists — the kinds of viruses we prepare for globally, even if they’re local now. That kind of recognition helps channel research funding, develop vaccines, train epidemiologists, and build lab capacity in endemic areas. But attention is uneven. The virus comes and goes, and when it goes quiet for a few years, so does the world’s interest.
That’s the dangerous lull — because Machupo doesn’t vanish. It waits. In a field, in a wall, in a pile of spilled grain. It waits for the right conditions.
And that’s why prevention is never really done. It’s not a one-time campaign. It’s a constant negotiation with the ecosystem, a long-term investment in housing, education, infrastructure, and surveillance. It’s about community resilience, not just viral containment.
So when we talk about prevention, we’re not just talking about avoiding illness. We’re talking about preserving normalcy, protecting the vulnerable, and staying one step ahead of the invisible.
Recent Developments (2025–2026)
Machupo virus doesn’t make headlines often. It doesn’t travel fast like influenza or provoke global panic like Ebola. It stays tucked away — geographically, virologically, politically. But that doesn’t mean nothing’s happening.
In fact, if you’ve been paying attention these last two years, you’ll know something has shifted. The virus is speaking up again — and this time, people are listening.
Let’s start with the 2025 outbreak near San Ramón, in the Beni Department. It began quietly: a cluster of unexplained hemorrhagic illnesses among agricultural workers clearing new plots of land for soy farming. At first, it looked like leptospirosis, maybe yellow fever. But then came the bleeding, the seizures, the high fevers unresponsive to standard care. By the time samples were flown out and tested in La Paz, Machupo virus was confirmed. At least 18 people were infected, five of whom died — including a nurse who had cared for multiple patients before anyone realized what they were dealing with.
This wasn’t a huge outbreak by pandemic standards. But it was significant.
Why?
Because it happened in a region not previously seen as high risk. Because it crossed into a community with a small airstrip and regular contact with Brazil. Because genomic sequencing of the virus revealed slight but meaningful mutations in the glycoprotein region — the same part that allows the virus to enter human cells.
That’s not to say Machupo is mutating into a more dangerous form. But it is adapting, subtly, over time — and that has researchers asking urgent questions:
Are these changes affecting transmission? Could they impact future vaccine development? Are we seeing early signs of a virus preparing for a broader ecological shift?
In parallel, 2026 brought a glimmer of good news from the world of science.
A collaborative team from Bolivia’s Instituto Nacional de Laboratorios de Salud (INLASA) and a European virology consortium completed a phase I trial of a Machupo vaccine candidate using an mRNA platform — yes, the same backbone used for COVID-19 vaccines. Early data suggest strong antibody responses in primates and acceptable safety profiles in healthy adult volunteers.
No, it’s not ready yet. But it’s further than we’ve been in decades.
At the same time, diagnostic tools are quietly getting faster and more accessible. Portable RT-PCR units — once limited to elite labs — are now being deployed in regional health centers with solar backup power. That means earlier detection, better containment, and a fighting chance at using antivirals like ribavirin before the virus tips the patient into irreversible shock.
There’s also momentum building around point-of-care antigen tests — cheap, rapid, paper-strip-style devices that can flag Machupo infection at the bedside in under 30 minutes. These are in late-stage field validation right now, and if they prove reliable, they could become game-changers for rural clinics that currently operate with more guesswork than gear.
The science is advancing—but the question now is how fast we can move that science from the lab to the people.
In another post called “Infectious Disease Preparedness”, we offer insights that complement this article. It dives into what readiness really looks like in the age of emerging viruses—and what happens when readiness is more theory than practice.
But even with these advances, one truth remains clear: none of this matters if it doesn’t reach the people who need it most. A vaccine sitting in a lab freezer doesn’t protect the woman hauling corn in a rodent-infested granary. A diagnostic tool that costs $400 isn’t useful to a health post running on diesel and duct tape. The gap between scientific progress and rural implementation is still wide — and if we don’t bridge it, people will keep dying preventable deaths.
So yes, Machupo virus is getting more attention. Yes, tools are being sharpened. But the real test won’t be whether we can make the tech — it will be whether we can deliver it, train people to use it, and build systems that don’t fall apart when the next outbreak hits.
And if the past two years have taught us anything, it’s this: the virus isn’t standing still. Neither should we.
Machupo Virus Q&A
1. What is the Machupo virus and why is it significant in Bolivia?
Machupo virus is a member of the Arenavirus family that causes Bolivian Hemorrhagic Fever (BHF), a severe and often fatal disease. It was first identified in the 1950s in northeastern Bolivia and is named after the Machupo River region where initial outbreaks occurred. It remains a significant public health concern in Bolivia due to its high fatality rates and potential for outbreaks.
2. What are the genetic characteristics of Machupo virus and how does it infect cells?
Machupo virus has a bisegmented RNA genome with ambisense coding, meaning it efficiently encodes proteins on both RNA strands. It infects cells by binding its glycoprotein spikes to host receptors (notably alpha-dystroglycan), entering via endocytosis, then replicating inside the host cell. It evades the immune system by suppressing interferon production, allowing rapid viral replication.
3. Where is the Machupo virus found and how does it spread from rodents to humans?
The virus is endemic primarily in Bolivia’s Beni Department, living in rodent reservoirs—mainly the Calomys callosusmouse. Humans are infected through aerosolized virus particles from rodent urine, feces, or saliva, often via inhalation or contact with contaminated materials in rural settings.
4. Can Machupo virus be transmitted between humans?
Yes, though less commonly. Human-to-human transmission occurs through direct contact with infected bodily fluids like blood, saliva, or vomit, particularly endangering healthcare workers and caregivers without proper protective measures.
5. What are the symptoms and progression of Bolivian Hemorrhagic Fever?
Symptoms start like a flu — fever, headache, muscle aches — but progress to severe hemorrhagic manifestations, including bleeding from gums and nose, internal bleeding, neurological signs like confusion or seizures, and multi-organ failure. The disease progresses rapidly, often within 7-14 days after exposure, with mortality rates historically around 20-30%.
6. How is Machupo virus infection diagnosed and what are the challenges?
Diagnosis relies on clinical suspicion and laboratory confirmation via molecular methods like RT-PCR, which detect viral RNA. Serological tests (ELISA) can detect antibodies or viral antigens. Challenges include symptom overlap with other tropical diseases, limited lab access in endemic regions, and the need for high biosafety facilities for handling samples.
7. What treatment options are available for Machupo virus infection?
No specific cure exists. Treatment is primarily supportive — managing fluids, electrolytes, bleeding, and organ function. Ribavirin, an antiviral, has shown some effectiveness if given early but is not widely available or proven specifically for Machupo. Experimental therapies like monoclonal antibodies are under research but not yet in clinical use.
8. Is there a vaccine for Machupo virus and what is the status of vaccine development?
No licensed vaccine is currently available. Past experimental vaccines showed promise but were never fully developed or approved. Recent efforts, including mRNA vaccine candidates tested in early human trials, show promise but are still years away from general availability.
9. How can Machupo virus outbreaks be prevented and controlled?
Prevention focuses on reducing contact with infected rodents through rodent control, improving housing, safe food storage, community education, and environmental hygiene. During outbreaks, isolation of cases, proper use of personal protective equipment (PPE) by healthcare workers, contact tracing, and public health messaging are essential.
10. What recent outbreaks and scientific developments have occurred between 2025 and 2026?
Recent outbreaks in new areas of Bolivia, including clusters linked to agricultural expansion, have raised concerns about viral adaptation. Scientific progress includes early-stage trials of mRNA vaccines, deployment of portable molecular diagnostics, and development of rapid antigen tests, though implementation in rural endemic areas remains limited.
Conclusion
The Machupo virus may fly under the radar in global headlines, but its impact is deeply felt where it matters most—in the lives of people living in rural Bolivia. This virus, quietly harbored in native rodents, teaches us how fragile the balance is between humans and nature, and how quickly that balance can tip toward danger.
We’ve seen how Machupo cleverly invades the body, evades immune defenses, and causes a disease that can be swift and deadly. Yet, the challenges extend far beyond the biology: diagnosing the infection early is difficult, treatment options are limited, and prevention requires addressing social, economic, and environmental realities.
Recent scientific advances offer hope—new vaccines, faster diagnostics, and better understanding of the virus—but these breakthroughs must be matched by real-world access and strong public health systems. Otherwise, they risk staying locked away in laboratories, far from the communities that need them.
Machupo reminds us that combating infectious diseases is not just about fighting pathogens; it’s about building resilient health infrastructures, educating and empowering communities, and respecting the ecosystems we share.
As we look ahead, preparedness and investment remain our best defenses. Machupo virus is not just a Bolivian story—it’s a global lesson in vigilance, science, and compassion.










