Avian Influenza H5N1: A Persistent Zoonotic Threat
Introduction
Avian Influenza H5N1. Just hearing the name might stir a vague memory of alarming headlines and bird culling operations. But what exactly is H5N1, and why does it keep popping up in conversations about public health?
Let’s start at the beginning.
History of H5N1 Outbreaks
The H5N1 strain of avian influenza first made global headlines in 1997, when it crossed the species barrier in Hong Kong. Eighteen people were infected, and six of them died—a shocking case fatality rate that immediately signaled this wasn’t just another flu. Authorities took drastic action, culling over a million chickens in an effort to contain the virus. It worked, for a while.
But H5N1 didn’t disappear. It retreated into the wild and domestic bird populations, resurfacing sporadically in Asia, Europe, and Africa over the years. From 2003 onward, it became endemic in some regions, occasionally infecting humans who had close contact with poultry.
Why couldn’t we just eliminate it altogether? What makes this virus so stubbornly persistent?
Part of the answer lies in how it spreads and mutates. H5N1 circulates widely among birds—especially ducks and chickens—without always causing noticeable illness. That means it can move undetected, jumping across farms, borders, and species.
Significance in Public Health
You might wonder: “If this virus has been around for decades, and human cases are rare, is it really still a threat?”
Fair question. Here’s the issue: when H5N1 does infect humans, it tends to be severe. The World Health Organization (WHO) reports a case fatality rate of around 50% in confirmed human infections. That’s extraordinarily high compared to seasonal flu, which usually kills less than 0.1% of those infected.
Even though human-to-human transmission is rare, the real danger is potential. What if the virus mutates in a way that makes it easily transmissible between people? That’s the nightmare scenario: a flu strain with the lethality of H5N1 and the contagiousness of regular influenza. It hasn’t happened yet—but virologists and public health officials keep a close watch because viruses like this evolve constantly.
So we’re left with a tricky balancing act. We don’t want to overreact to a threat that hasn’t fully materialized. But we also can’t afford to be complacent.
And that brings us to a key question for today’s world:
Are we prepared for a virus that’s already shown it can jump to humans—and may be only a few mutations away from spreading between us?
The unease around H5N1 isn’t just about past outbreaks—it’s about potential. The potential for a rare virus to jump species, mutate, and find new ways to spread. This same forward-looking concern is what’s driven attention toward Disease X—a placeholder for the next unknown pathogen that could emerge under just the right (or wrong) circumstances. H5N1 may not be Disease X, but it fits the mold unsettlingly well.
That’s the lens through which we’ll explore H5N1 in the sections ahead. From its virology to prevention strategies, this isn’t just a scientific curiosity—it’s a real and persistent challenge for global health.
Virology
Let’s get under the hood of the H5N1 virus. What makes this particular strain of avian influenza so dangerous? And why does it keep grabbing the attention of scientists, despite its relatively low number of human cases?
Understanding the virus’s makeup helps explain why it’s such a persistent—and potentially deadly—threat.
Characteristics of the H5N1 Virus
First things first: H5N1 is a type A influenza virus. That means it belongs to the same category as the seasonal flu viruses we see every year, but it’s a very different beast.
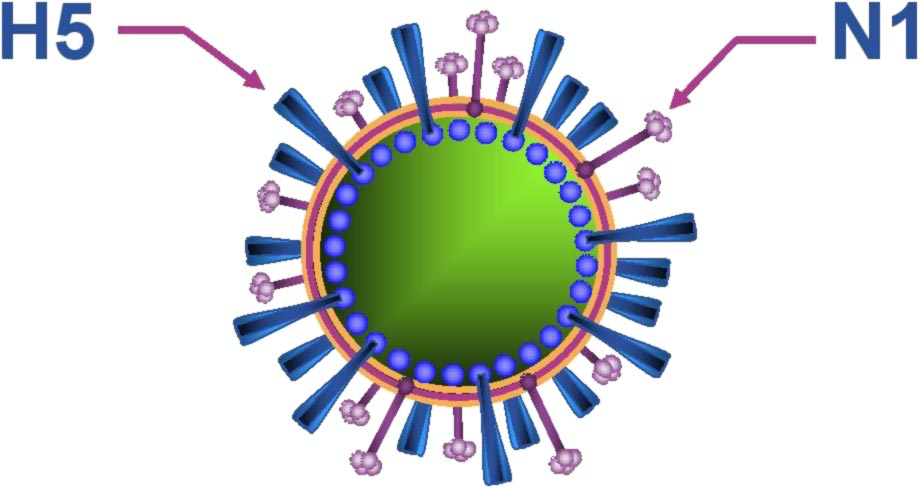
The name “H5N1” refers to the two proteins on the surface of the virus:
- Hemagglutinin (H5), which helps the virus bind to and enter host cells.
- Neuraminidase (N1), which helps it exit infected cells and spread to others.
There are 18 known hemagglutinin (H) and 11 neuraminidase (N) subtypes, which combine in various ways. So H5N1 is just one of many possible combos—but this particular one has proven unusually good at causing severe disease in both birds and humans.
Here’s where it gets fascinating (and a little scary): H5N1 doesn’t behave like typical human flu. It tends to target deeper parts of the respiratory tract, leading to severe pneumonia, acute respiratory distress, and multi-organ failure. And because our immune systems have little to no natural defense against it, the results can be devastating.
Why does it cause such high fatality rates?
Part of the answer may lie in the body’s own overreaction. In some patients, H5N1 triggers a cytokine storm—a massive inflammatory response that ends up doing more harm than good. In other words, the immune system’s attempt to fight the virus actually contributes to the damage.
Mutation and Reassortment Potential
Viruses don’t sit still. Influenza A viruses are infamous for their ability to mutate rapidly. And H5N1 is no exception.
There are two key ways this virus evolves:
- Antigenic drift – Small, gradual changes in the virus’s genetic code as it replicates. These changes can accumulate over time, potentially making the virus more infectious or helping it evade immune detection.
- Antigenic shift – A more dramatic process, where the virus mixes genes with other influenza viruses—usually when multiple strains infect the same host, like a pig or a human. This process, called reassortment, can result in a brand-new virus with unpredictable characteristics.
Could H5N1 combine with a seasonal flu virus and become more contagious?
That’s the worry. If a person—or even a pig—gets infected with H5N1 and a human flu virus at the same time, the two could “swap parts” and produce a hybrid virus that spreads easily between humans but retains the severity of H5N1.
We’ve seen this sort of thing before. The 2009 H1N1 pandemic (also known as “swine flu”) was the result of such a reassortment event. It wasn’t particularly lethal, but it spread globally in a matter of weeks. What if the next reassortment event gives us a virus with H5N1’s virulence and pandemic potential?
Here’s a question worth asking:
What can we do now to monitor or even predict when such a shift might occur?
That’s one reason why flu surveillance in both humans and animals is so critical—and why H5N1 keeps showing up on the radar of virologists and epidemiologists.
Transmission
It’s one thing for a virus to exist quietly in wild birds. It’s quite another when it begins infecting humans. So how does H5N1 actually make the leap from flocks to people—and could it eventually spread from person to person?
Let’s dig into how this virus moves, and why its transmission patterns keep scientists and public health officials on alert.
Zoonotic Transmission from Birds to Humans
First, let’s talk about the bird-to-human route. This is where most H5N1 infections have come from so far. But it’s not as simple as “a bird coughs, and someone gets the flu.” In fact, birds don’t usually cough—and H5N1 doesn’t spread through the air the way human flu does.
So how does it happen?
Most human cases have been linked to close contact with infected poultry, particularly during activities like:
- Slaughtering or defeathering chickens.
- Cleaning cages or working in live bird markets.
- Handling sick or dead birds without protective gear.
In these environments, the virus can be aerosolized in droplets, contaminating surfaces, tools, clothing, and even the air in enclosed spaces. If someone touches their face, breathes in contaminated dust, or gets the virus in their eyes, they can become infected.
It’s worth noting that not all exposed individuals get sick—but when they do, it tends to be serious. This raises questions about host susceptibility, immune response, and possibly even genetic factors that make some people more vulnerable than others.
Here’s a question to ponder:
How many mild or asymptomatic H5N1 infections go completely undetected?
It’s hard to say. Limited testing and the focus on severe cases mean we might be missing a larger picture of how often H5N1 crosses into humans with little consequence.
Human-to-Human Transmission Risks
This is the big one—the elephant in the room.
So far, sustained human-to-human transmission of H5N1 has not occurred. That’s a huge relief. But there have been a handful of isolated cases where limited person-to-person spread was suspected—typically among family members in close quarters.
For example, in 2006, a family cluster in Indonesia suggested the possibility of limited human transmission, likely through prolonged, close, and unprotected exposure. But even then, the virus didn’t spread beyond that group.
What’s stopping it from spreading like seasonal flu?
Mainly, it’s a receptor issue. Human flu viruses bind to alpha-2,6 sialic acid receptors, which are abundant in the upper respiratory tract (think nose and throat). H5N1, however, prefers alpha-2,3 receptors, found deeper in the lungs and more common in birds.
That makes it harder for H5N1 to establish an infection in humans—and much harder to transmit via coughing, sneezing, or casual contact. But here’s the catch: viruses evolve.
What if H5N1 mutates to bind more efficiently to human receptors?
This is the scenario that virologists fear most. A few small genetic tweaks could, in theory, make H5N1 better suited for human-to-human spread. And because of its high mortality rate, that could be catastrophic.
Another concern is dual infection—if someone is infected with both H5N1 and a human-adapted flu strain, the two viruses could reassort, combining high transmissibility with severe pathogenicity.
Could a single mutation make the difference between an isolated case and a pandemic?
We don’t know yet. But research using animal models (like ferrets) suggests that it might not take much.
And that’s why every new outbreak, every new cluster of cases, is treated with urgency. Surveillance, genomic sequencing, and field investigations are all critical to catch signs of adaptation early.
Transmission may seem like a technical topic, but it’s the crux of the threat. Right now, H5N1 remains mostly a zoonotic virus with limited human spread—but that balance is precarious. Understanding how and why the virus moves between species isn’t just a matter of curiosity—it’s a potential early warning system for the next pandemic.
Clinical Manifestations
When H5N1 infects a human, the experience is often far more intense than anything we typically associate with “the flu.” Unlike the seasonal strains we’ve grown used to navigating with rest, fluids, and the occasional sick day, H5N1 can unfold like a medical emergency—and in many cases, it is one.
It’s this potential for severity, unpredictability, and rapid deterioration that puts H5N1 in a league of its own. So what exactly does the clinical picture look like when the virus takes hold?
Symptoms in Humans
In the earliest stages, H5N1 doesn’t necessarily announce itself as anything unusual. In fact, its initial symptoms—fever, cough, sore throat, muscle aches, fatigue—are all too familiar. These are the same early warning signs of seasonal flu or even a bad cold. That overlap is one of the virus’s most dangerous traits: it hides in plain sight.
For a doctor or patient looking at these symptoms in isolation, there’s rarely a flashing red light saying, “This is different.” But under the surface, something much more aggressive is brewing.
Within just a few days, many patients transition from moderate discomfort to a rapid and terrifying decline. Respiratory symptoms intensify. Breathing becomes labored. Chest pain sets in. Coughs may become productive, tinged with blood, and accompanied by the unmistakable signs of lower respiratory tract infection. What began as “flu-like symptoms” can quickly escalate into viral pneumonia and, in more severe cases, acute respiratory distress syndrome (ARDS). This is not merely a complication—it’s a tipping point, often requiring mechanical ventilation and intensive care.
But the lungs aren’t the only organs under siege. H5N1 is capable of triggering a wide-reaching assault on the body:
- Gastrointestinal distress is reported in many cases, with diarrhea, nausea, and abdominal pain occurring early—sometimes even before respiratory symptoms dominate.
- Neurological effects such as seizures, confusion, or loss of consciousness hint at a virus that may be crossing into the central nervous system.
- Multi-organ dysfunction, including kidney and liver failure, can occur as the body spirals into systemic inflammation and collapse.
This raises a crucial—and haunting—question:
Why does a virus that begins in the lungs end up disrupting so many other systems?
The answer lies in a combination of direct viral damage and the body’s own overreaction. H5N1 doesn’t just replicate in respiratory tissue—it can also infect cells in the intestines, brain, and beyond. Meanwhile, the immune system, in its desperate attempt to fight the invader, often unleashes a cytokine storm—an overwhelming flood of inflammatory molecules that, rather than controlling the infection, damage the body’s own tissues. It’s the biochemical equivalent of friendly fire.
In some ways, the immune system’s response becomes more dangerous than the virus itself.
Severity and Case Fatality Rates
All of this adds up to one harsh reality: H5N1 infections in humans are not only severe—they are often deadly.
The case fatality rate hovers around 50%, based on confirmed cases. That’s not a typo. Half of the people officially diagnosed with H5N1 have died from it. For comparison, seasonal flu kills less than one in a thousand. Even pandemic strains like H1N1 (2009) had fatality rates far below 1%.
There is ongoing debate about whether that 50% figure paints the full picture. It’s entirely possible—likely, even—that mild or asymptomatic infections go unrecognized and unreported. But even if the “true” fatality rate were significantly lower, H5N1 would still rank among the most dangerous influenza viruses known to infect humans.
And severity isn’t just about how many die—it’s also about how quickly things go wrong.
In many cases, patients deteriorate rapidly within 48–72 hours of initial symptoms. Hospitalization is often required within days. Intensive care, ventilatory support, and aggressive interventions follow. Even then, many do not survive.
There’s another troubling layer: H5N1 doesn’t only strike the old or frail. While chronic illness and age can certainly increase vulnerability, this virus has proven capable of taking the lives of young, previously healthy adults and children. That echoes the pattern seen during the 1918 influenza pandemic, where a virulent strain hit hardest not the weakest, but the strongest—those with robust immune systems that, ironically, mounted the most damaging inflammatory responses.
So we must ask ourselves:
- Would we recognize H5N1 if it showed up in a clinic tomorrow?
- Would we think “bird flu,” or just assume it’s another tough winter bug?
The answer, unfortunately, depends on awareness. And that’s what makes H5N1 so risky in clinical settings—it doesn’t always look different at first glance. The danger lies in delay: every hour spent misdiagnosing or underestimating can mean the difference between recovery and death.
Clinicians need to be aware. Public health systems need to be vigilant. And the public needs to understand that while H5N1 is rare, it is not theoretical.
Recognizing the full scope of clinical manifestations is more than a diagnostic task—it’s the foundation of prevention, treatment, and outbreak containment.
Early detection is the linchpin of outbreak response—but spotting a dangerous virus in a sea of similar symptoms is no easy task. This challenge isn’t unique to infectious disease, either. In fact, there are parallels in how subtle biological shifts are tracked elsewhere in medicine. Take CBC and Cancer Detection, where basic blood tests can reveal hidden malignancies if interpreted with the right tools and context. It’s a reminder that diagnostics are only as good as the systems—and minds—that use them.
And now that we understand how H5N1 affects the human body, the next step is figuring out how we can detect it early and accurately—before it’s too late.
Diagnosis
Knowing what H5N1 can do to the body is only half the battle. The other half—and arguably the trickier one—is identifying it early enough to make a difference. Diagnosis is the hinge point: everything from treatment decisions to outbreak response depends on the clarity, accuracy, and timing of that first clinical suspicion.
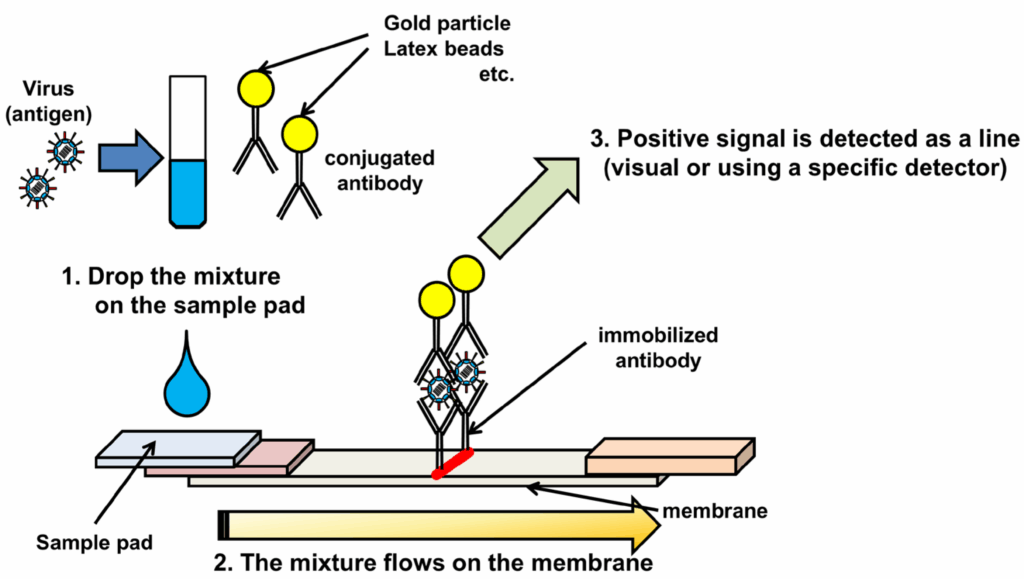
So how do we actually diagnose a virus that, early on, behaves like the flu… but can turn deadly in days?
Laboratory Testing and Imaging
The reality is, H5N1 can’t be diagnosed on symptoms alone. At least not reliably. As we explored earlier, its early signs—fever, cough, sore throat, malaise—are shared by dozens of respiratory viruses, from seasonal flu to RSV to SARS-CoV-2.
So clinicians need tools. And fast ones.
The gold standard for confirming H5N1 infection is reverse transcription polymerase chain reaction (RT-PCR), a lab technique that detects the virus’s genetic material with high sensitivity and specificity. These tests are usually performed on nasopharyngeal swabs, throat swabs, or in more severe cases, lower respiratory tract specimens like sputum or bronchoalveolar lavage.
But there’s a catch: RT-PCR testing for H5N1 isn’t always part of standard panels in most hospitals. It often requires sending samples to reference laboratories or national influenza centers, which adds valuable hours—or even days—to the diagnostic timeline.
And that leads to a pressing question:
What happens during those hours of waiting, especially if the patient is deteriorating?
That’s where clinical judgment and a detailed exposure history become crucial. If a patient presents with flu-like symptoms and has recently:
- Handled or been near poultry
- Traveled to or lived in a region with known outbreaks
- Had close contact with someone who died of a respiratory illness
… then H5N1 should jump much higher on the diagnostic radar.
While awaiting test results, imaging studies often help support a working diagnosis. Chest X-rays or CT scans typically reveal bilateral infiltrates, ground-glass opacities, or signs of viral pneumonia and ARDS. These findings aren’t specific to H5N1, but they do paint a picture of rapidly progressive lung damage—especially in younger, previously healthy patients. When combined with a high index of suspicion, this pattern can help trigger timely isolation, treatment, and alerting of public health authorities.
So we come to a tough question:
Are we relying too heavily on perfect test results, when time is the most limited resource in H5N1 management?
5.2 Challenges in Early Detection
Diagnosis isn’t just a medical challenge—it’s a systemic one.
One major barrier is access. Not every clinic or hospital—especially in rural or low-income regions—has immediate access to the tools needed to confirm H5N1. That’s troubling, given that many outbreaks start in precisely those settings: places where poultry is a daily part of life, where humans and birds live in close quarters, and where the virus has more chances to jump species.
And even where tests are available, awareness can lag behind the virus. In non-outbreak periods, clinicians may not consider avian flu at all—especially when the patient has no obvious exposure or when the region hasn’t seen a case in years. This leads to delays, misdiagnoses, and sometimes, failure to isolate patients who may be contagious.
Another issue is surveillance fatigue. With multiple emerging pathogens demanding attention—COVID-19, new influenza strains, dengue, and others—it’s easy for H5N1 to fade from the radar. Until it doesn’t.
So we have to ask:
- Should H5N1 testing be more routine in severe flu-like illnesses, especially in high-risk settings?
- Would rapid, point-of-care tests make a difference, or do they risk overdiagnosis and panic?
There’s no easy answer, but one thing is clear: we’re walking a diagnostic tightrope. On one side is the risk of over-testing and overreacting. On the other is the greater danger—missing the warning signs entirely.
Diagnosis, in the case of H5N1, isn’t just about naming a virus. It’s about detecting a threat in time to stop its spread, tailoring treatment before irreversible damage sets in, and mobilizing a response before a local infection becomes a global problem.
We’ll explore treatment strategies next—but as with most viral threats, the first and most powerful intervention is knowing what you’re dealing with. And doing it fast.
Treatment
So let’s say the diagnosis is in. H5N1 confirmed. The patient is symptomatic, possibly deteriorating. Now comes the most urgent question of all:
What can we actually do to treat it?
Unlike bacterial infections, where a simple course of antibiotics might do the trick, viruses are notoriously harder to treat. And when it comes to H5N1, the options are limited, the evidence is evolving, and the clock is always ticking. But there are treatments—and when administered early enough, they can make a real difference.
Antiviral Medications and Their Effectiveness
The frontline weapon against H5N1 infection is a class of drugs called neuraminidase inhibitors. These include:
- Oseltamivir (commonly known by the brand name Tamiflu)
- Zanamivir (inhaled, known as Relenza)
- Peramivir (intravenous formulation)
- And in some settings, the newer baloxavir marboxil, although it works through a different mechanism and its use in H5N1 remains under investigation.
Of these, oseltamivir is the most commonly used. It works by blocking the neuraminidase enzyme, essentially trapping new viral particles inside infected cells and preventing further spread. The earlier it’s given—ideally within the first 48 hours of symptom onset—the better the outcomes tend to be.
But here’s the tricky part:
Most patients don’t show up early.
H5N1 symptoms escalate fast, and by the time someone is sick enough to be hospitalized and tested, the virus may have already replicated extensively. That doesn’t mean antivirals are useless late in the game—but their effectiveness can diminish over time, especially if the virus has already overwhelmed the lungs or triggered a cytokine storm.
Some strains of H5N1 have also shown partial resistance to oseltamivir, which adds another layer of uncertainty. Surveillance of viral mutations remains essential to guide drug selection, and in some cases, doctors might need to use higher doses or longer courses than those used for seasonal flu.
This leads to a natural question:
Should we be stockpiling more diverse antivirals, or developing entirely new classes to outpace evolving resistance?
It’s a question that goes beyond H5N1, but the urgency it presents here is very real.
Supportive Care and Management of Complications
Even with antivirals, medication alone rarely does the job. Much of the treatment for H5N1 is what physicians call supportive care—not because it’s secondary, but because it’s about sustaining life while the body fights back.
Respiratory support is often the most critical aspect. Many patients with H5N1 develop severe viral pneumonia or ARDS, which may require:
- Supplemental oxygen, initially via nasal cannula or mask.
- Non-invasive ventilation (e.g., BiPAP or CPAP) if oxygen levels remain low.
- Mechanical ventilation in intensive care settings if respiratory failure progresses.
- In extreme cases, extracorporeal membrane oxygenation (ECMO)—a machine that oxygenates blood outside the body, essentially giving the lungs time to rest and heal.
Fluid management becomes a delicate balance. Patients often experience shock, either due to sepsis or massive inflammation, but overhydration can worsen pulmonary edema. Careful monitoring of kidney function, liver enzymes, and coagulation status is crucial, especially when multi-organ dysfunction sets in.
Antibiotics may be used, not to treat the virus, but to cover secondary bacterial infections—a common complication of viral pneumonia that can turn fatal if overlooked. In some cases, steroids or immunomodulatory therapies are considered, but their role in H5N1 remains controversial and must be weighed against potential risks.
One might wonder:
If treatment is so complex, how feasible is it in regions with limited ICU capacity?
The honest answer is—not very. And that’s part of what makes H5N1 such a global health concern. In lower-resource settings, even with antivirals on hand, the absence of ventilators, trained staff, or monitoring equipment can be the difference between life and death.
That’s why international preparedness matters. Outbreak control isn’t just about having the right drugs—it’s about having the right systems in place to deliver comprehensive care, rapidly and equitably.
The Psychological Weight of Treatment Decisions
There’s also a human side to this—something harder to quantify but impossible to ignore.
For clinicians treating a confirmed H5N1 case, the stakes are unusually high. Every decision feels amplified. Do you intubate now, or wait one more hour? Do you administer high-dose antivirals even if evidence is thin? Is the family prepared for what might come next?
These are emotionally draining scenarios. And if cases ever become more common—if H5N1 gains transmissibility—this stress will multiply across emergency departments and ICUs around the world.
So we have to ask:
Are we training clinicians not just in the medical protocols, but in the mental and ethical resilience needed to navigate a disease like H5N1?
It’s a reminder that treatment isn’t just about science—it’s also about courage, capacity, and compassion under pressure.
In the next section—Prevention—we’ll shift gears from response to readiness. Because in truth, the best treatment for H5N1 is to never get it in the first place.
Prevention
If treatment is the fire extinguisher, prevention is the firewall—and in the case of H5N1, you want that wall reinforced, monitored, and never taken for granted. Because once human infection begins, options narrow quickly. The virus moves fast. Outcomes are uncertain. But before that point, we have opportunities—real, tangible ones—to reduce risk at every level.
And prevention, in the world of H5N1, starts not in hospitals, but in barns, wetlands, and farms.
Biosecurity Measures in Poultry Farming
Let’s face it: the frontlines of H5N1 prevention aren’t in medical clinics—they’re in chicken coops.
Poultry, particularly domestic birds like chickens and ducks, are the primary reservoir for H5N1. This virus doesn’t need humans to survive—it thrives in birds, and migratory wild species help spread it across continents. When it reaches domesticated poultry, the real danger begins, because that’s when people start to come into close contact with infected animals.
So what can be done?
Biosecurity is the cornerstone of avian flu prevention in agriculture. That means controlling access to farms, separating wild birds from domestic flocks, disinfecting equipment, limiting visitors, and using personal protective gear when handling birds. It also involves quickly identifying and isolating infected animals—and in many cases, culling entire flocks to prevent the virus from spreading.
Yes, culling is controversial. It’s economically devastating to farmers and emotionally painful. But in the absence of perfect vaccines or antiviral treatment for birds, it’s often the fastest way to contain an outbreak.
That brings us to a hard but necessary question:
How do we balance animal welfare, economic survival, and public health when facing a rapidly spreading zoonotic threat?
It’s not just a technical issue—it’s a political and ethical one, especially in lower-income regions where poultry farming is tied closely to food security and livelihoods.
But there’s no doubt: effective biosecurity, even when costly, is far cheaper than a human outbreak.
Vaccination Strategies for Birds and Humans
The next pillar of prevention is vaccination. And here, things get more complex.
For birds, several types of vaccines exist—primarily inactivated or recombinant versions targeting various H5 strains. When deployed correctly, they can help control outbreaks in poultry, reduce viral shedding, and minimize human exposure. But it’s not a silver bullet.
Vaccinating birds requires:
- Accurate strain matching
- Large-scale manufacturing
- Logistical coordination
- Monitoring for vaccine escape mutants—viruses that evolve to sidestep immunity
Some countries use mass poultry vaccination as a core strategy. Others avoid it, fearing it might mask outbreaks and allow asymptomatic carriers to spread the virus unnoticed. It’s a double-edged sword: vaccinate too little, and outbreaks rage; vaccinate too widely without surveillance, and the virus hides in plain sight.
Now, what about people? Should humans be vaccinated against H5N1?
Currently, there is no H5N1 vaccine in routine human use. However, prototype vaccines have been developed and stockpiled in many countries, particularly as part of pandemic preparedness programs. These include monovalent inactivated vaccines and newer platforms like mRNA-based candidates, modeled after the technology used for COVID-19.
The idea is simple: if H5N1 ever becomes a pandemic threat, we want to be ready to roll out vaccines fast. But readiness and reality are two different things. Stockpiling is expensive. Shelf life is limited. Strain selection is uncertain. And perhaps the biggest question:
How do you prepare millions of doses for a virus that may never mutate—or may mutate tomorrow?
It’s a logistical and strategic gamble. But as the world learned with SARS-CoV-2, having a head start matters.
There’s also discussion around vaccinating high-risk human populations, such as poultry workers, veterinarians, and wildlife handlers. The argument is compelling: these are the people most likely to encounter H5N1 first. But even here, decisions are shaped by cost, availability, and risk-benefit analyses that differ by region.
So we have to ask:
Should we be more proactive in vaccinating select groups, even if human transmission remains rare? Or wait until the virus gives us a clear reason to act?
There are no easy answers—but history shows that viruses don’t wait for bureaucracy.
Prevention Is Global, Not Local
One of the most important lessons from past outbreaks—avian flu, swine flu, COVID-19—is that pathogens don’t respect borders. A poultry outbreak in Vietnam can lead to human infections in Cambodia and policy changes in France. Surveillance and prevention must be coordinated globally.
Organizations like the World Health Organization (WHO), the Food and Agriculture Organization (FAO), and the World Organisation for Animal Health (WOAH) collaborate through platforms like One Health to monitor and manage zoonotic diseases across species and systems. But funding is patchy. Coordination is inconsistent. And geopolitical tensions can slow response times.
We’re left with another big-picture question:
Can the world build sustainable, cooperative systems for zoonotic disease prevention—or are we doomed to keep playing catch-up?
Preventing H5N1 isn’t about eliminating one virus. It’s about maintaining a fragile equilibrium between humans, animals, and ecosystems. Every broken link in that chain—be it a neglected farm, an ignored outbreak report, or a delayed policy—becomes a point of vulnerability.
The next section will take us into Recent Developments, where we’ll examine how H5N1 has evolved in just the past year or two—and what it might be trying to tell us about what comes next.
Recent Developments (2025–2026)
If you’ve been following the headlines recently, you might have noticed a worrying trend: H5N1 is back in the news. Not just as a theoretical risk or an occasional poultry outbreak—but as an active, evolving presence in parts of the world that haven’t historically been on the avian influenza map.
What’s changed? And more importantly—what does this change mean?
Let’s look at the recent developments in detail, because they may be shaping the next chapter in this virus’s unpredictable story.
Outbreaks in Brazil and India
In early 2025, Brazilian health authorities reported a cluster of suspicious poultry deaths in the state of Paraná, one of the country’s major agricultural hubs. Within days, laboratory testing confirmed the presence of highly pathogenic avian influenza H5N1. What made this especially concerning wasn’t just the location—it was the speed and scale of the spread.
Brazil, a leading exporter of poultry meat, had never before reported a major H5N1 incursion. For the virus to leap from its usual strongholds in Asia and parts of Africa to South America marks a geographic shift that demands close attention.
Less than two months later, similar headlines emerged from India, where multiple states—West Bengal, Kerala, and Punjab among them—reported unusual mortality in backyard and commercial poultry. In a handful of cases, human exposure was confirmed. One child in Kerala was hospitalized with severe respiratory symptoms following contact with infected birds. The case didn’t result in death, but it reignited public fears and drew comparisons to earlier fatal outbreaks.
These events raise several red flags:
- H5N1 is spreading more widely, not just in terms of geography but also ecological range.
- Surveillance systems in both nations caught the outbreaks, but human exposure occurred despite warnings.
- Global trade and migratory bird routes may be accelerating viral movement between continents.
So here’s a critical question:
Are we witnessing a virus quietly expanding its territory, waiting for the right mutation—or are these scattered events just noise in a complex viral landscape?
We can’t say with certainty. But either possibility requires a serious response.
Genetic Studies Linking Cases in Different Regions
Here’s where things get really interesting—and a little unsettling.
Researchers at several leading virology centers, including the Institut Pasteur and the U.S. CDC, have recently sequenced samples from H5N1 outbreaks across continents. Using advanced genomic analysis, they’ve begun to notice patterns—shared mutations, genetic “signatures,” and structural similarities in viruses found thousands of miles apart.
For example, strains isolated in northern India in late 2025 bore striking resemblance to those found in southern Brazil. Not identical, but genetically related, suggesting either:
- A common origin, perhaps via migratory birds; or
- A convergent evolution, where the virus is adapting in similar ways across different environments.
Some of the mutations observed are associated with increased replication efficiency in mammalian cells, though not necessarily with airborne transmission. Still, any hint of mammalian adaptation raises the stakes.
And then there’s the discovery of a mutation cluster in the polymerase gene (PB2 E627K and others), which has previously been linked in lab models to enhanced virulence and transmissibility in mammals. It doesn’t mean H5N1 is now spreading easily between humans—but it does mean the virus is exploring genetic territory where that becomes more likely.
That leads us to a sobering question:
If H5N1 is mutating across regions in similar ways, are we seeing early signs of selective pressure toward human adaptation?
It’s too early to know for sure. But this is why global surveillance, transparent data sharing, and open-access genomics are more than academic tools—they are early warning systems.
What These Developments Might Be Telling Us
Outbreaks in new regions. Cross-continental genetic links. Mild but confirmed human cases. All of this points to a virus that isn’t dormant—it’s active, mutating, and probing boundaries.
So what might come next?
One possibility is that these are flare-ups that will die down, as many have before. Seasonal changes, containment measures, and culling programs might suppress transmission in birds, reducing spillover risk to humans.
Another possibility? The virus continues evolving under the radar—slowly adapting to mammals, occasionally infecting humans, and waiting for the moment when a single reassortment event or mutation unlocks efficient human-to-human transmission.
Are we being alarmist by even asking that question? Or are we being cautiously realistic, given what we know about viral evolution, zoonotic spillovers, and the last two decades of H5N1 history?
The answer likely lies somewhere in the middle.
The real lesson here is not to panic—but to pay attention. These recent developments aren’t a prediction, but a prompt. They’re telling us to invest in surveillance, to strengthen our diagnostic infrastructure, to revisit vaccination strategies, and to prepare—not just for if something happens, but for the moment it starts to.
Because if H5N1 has taught us anything, it’s that quiet outbreaks can become loud ones. And small genetic changes can have massive consequences.
The bigger picture with H5N1 is about systems: how they anticipate, detect, and respond. Interestingly, some of the same challenges show up in entirely different arenas—like neurodevelopmental health. Autism in 2025 explores how environmental, diagnostic, and social factors intersect in a very different kind of public health story, but the common thread is clear: early signals matter. Whether it’s a shift in viral behavior or a change in child development patterns, we ignore those signals at our peril.
Frequently Asked Questions (FAQ)
1. What is H5N1, and why is it different from seasonal flu?
H5N1 is a subtype of avian influenza A virus that primarily infects birds but can occasionally cross over to humans. Unlike seasonal flu, which spreads easily but is usually mild, H5N1 infections in humans are rare but often severe—with a case fatality rate of around 50%.
2. How do humans get infected with H5N1?
Most human cases result from direct contact with infected birds or contaminated environments, such as in poultry farms or live bird markets. Human-to-human transmission is extremely rare but not impossible under certain conditions.
3. What are the symptoms of H5N1 infection?
Early symptoms can resemble the flu: fever, cough, sore throat, fatigue. However, the disease can escalate rapidly to pneumonia, acute respiratory distress, and multi-organ failure. Gastrointestinal and neurological symptoms are also common in severe cases.
4. How is H5N1 diagnosed in humans?
Diagnosis relies on RT-PCR testing to detect viral RNA. Chest imaging often reveals pneumonia or ARDS. A patient’s history of exposure to birds is crucial in raising clinical suspicion early.
5. Is there a treatment for H5N1?
Yes. Antiviral drugs like oseltamivir can be effective, especially when started early. Most patients also require supportive care, including oxygen therapy or even intensive care. Delays in diagnosis and treatment can be fatal.
6. Can H5N1 be prevented in humans?
Prevention hinges on controlling outbreaks in birds through biosecurity measures, surveillance, and vaccination programs. For people at high risk—such as poultry workers—future vaccination may be considered if effective vaccines are approved.
7. Has H5N1 ever shown signs of spreading between humans?
Only in very limited, close-contact situations, such as among family members. There’s no sustained human-to-human transmission, but ongoing mutations in the virus warrant close monitoring.
8. Why are recent outbreaks in Brazil and India significant?
These outbreaks mark a geographic expansion of H5N1 into new territories and ecosystems. Genetic analyses suggest some of these viral strains may be adapting to mammals, underscoring the need for global vigilance.
9. Is the world prepared for an H5N1 pandemic?
Some progress has been made—vaccine prototypes, antiviral stockpiles, and international surveillance systems exist. But readiness remains uneven, especially in lower-resource regions. Prevention and early detection are still our best tools.
10. Should I be worried about H5N1 now?
Not panicked—but definitely aware. For most people, the risk remains very low. But for health authorities, veterinarians, and public health planners, now is the time to act, plan, and prepare—not after the fact.
Conclusion: Vigilance, Not Alarm
Avian influenza H5N1 is a virus that has spent over two decades hovering just outside the center of global attention. It’s not new. It’s not always urgent. But it is always there, quietly circulating in bird populations, occasionally flaring up in humans, and evolving in ways that remind us how closely human and animal health are intertwined.
What makes H5N1 so unique—and so worrisome—is not just its capacity for severe disease. It’s the combination of its lethality, its proximity to human life, and its potential for transformation. A virus that kills half its confirmed human hosts but has not yet learned how to spread from person to person is a biological paradox—and a public health time bomb.
But this story doesn’t have to end in crisis.
Every outbreak we catch early, every bird population we vaccinate, every farmer we educate, and every lab we fund for surveillance pushes us further from that tipping point. Prevention is not glamorous, and early intervention rarely makes headlines—but it saves lives.
So, what’s the real takeaway here?
H5N1 isn’t tomorrow’s threat. It’s today’s opportunity—an opportunity to build systems that prevent outbreaks, not just respond to them. To listen when nature whispers, not when it shouts.
Because in public health, as in so many things, the best crisis is the one you never have.