Candida Auris: The Emerging Multidrug-Resistant Fungus
Introduction
Overview of Candida auris as a Global Health Threat
Have you ever heard of a fungus that can sneak into hospitals, resist most of our antifungal weapons, and spread from patient to patient with alarming ease? Meet Candida auris—a microscopic organism that has quietly become one of the most unsettling infectious threats of the 21st century.
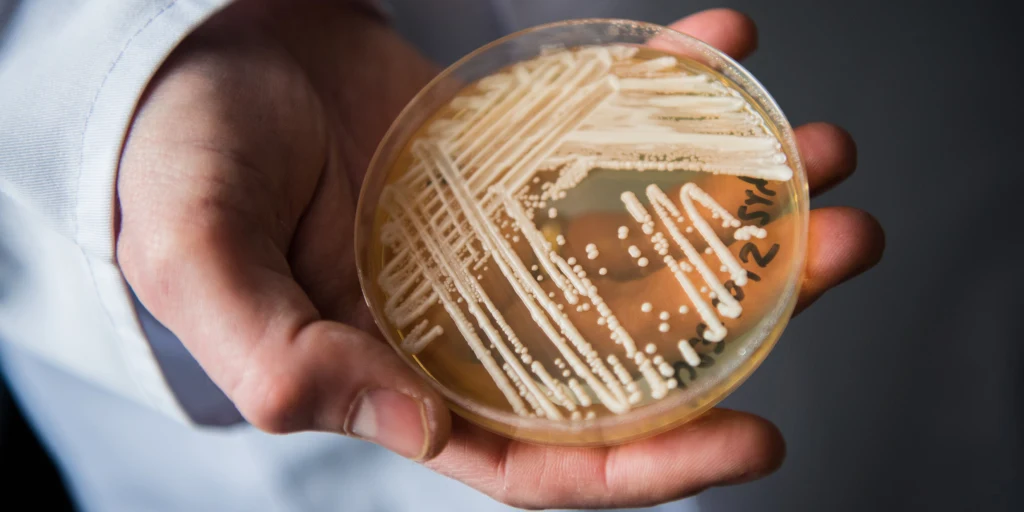
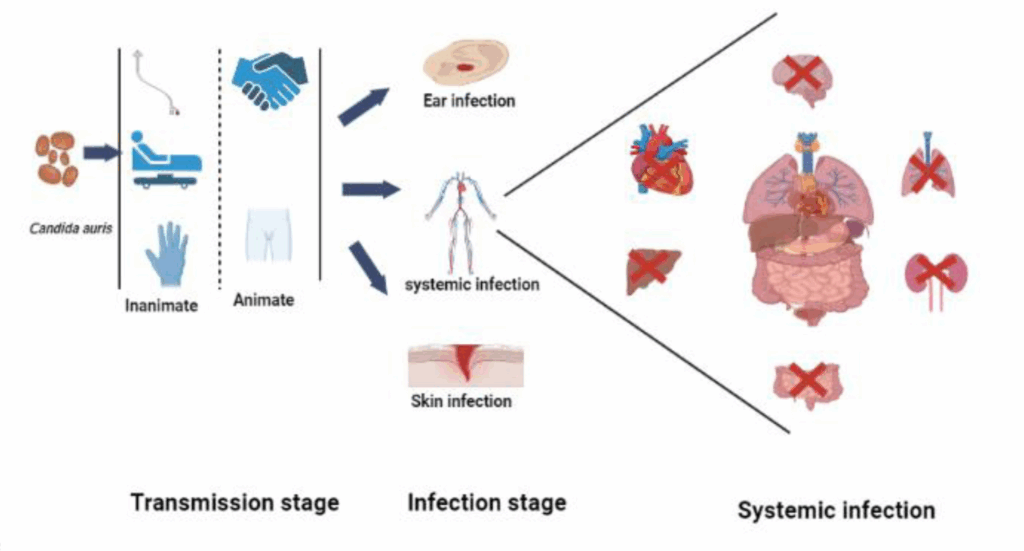
First identified in 2009 in Japan from a patient’s ear discharge—hence the species name auris—this fungus has since spread across more than 40 countries on six continents. What makes C. auris especially concerning isn’t just its global presence, but its resilience: it is often resistant to multiple classes of antifungal drugs, which severely limits treatment options.
So, why is the world’s health community sounding alarms over this seemingly obscure yeast? Because Candida auris is not just another hospital-acquired infection. It represents a new frontier in our ongoing struggle against antimicrobial resistance. Its emergence underscores how our current medical infrastructure—especially in hospitals and long-term care facilities—can be quickly overwhelmed by a novel, adaptable pathogen.
Emergence and Significance in Healthcare Settings
Why has Candida auris become such a pressing issue in hospitals and intensive care units? The answer lies in a perfect storm of factors: it’s hardy, elusive, and highly opportunistic.
Unlike most Candida species, C. auris is notorious for persisting on surfaces—such as bedrails, IV poles, and even medical equipment—for days or weeks. In fact, routine cleaning agents that work against other fungi may not be enough to wipe it out. This makes it exceptionally challenging to control once it infiltrates a healthcare environment.
Even more troubling is its ability to cause invasive infections, particularly in patients who are already critically ill. Individuals with central venous catheters, recent surgeries, or long-term antibiotic use are at greater risk. In such patients, C. auris can enter the bloodstream, leading to sepsis, organ failure, and, in some cases, death. Mortality rates associated with bloodstream infections can be as high as 30–60%.
Another alarming feature? Standard laboratory tests often misidentify C. auris as other Candida species—such as Candida haemulonii—leading to delays in appropriate treatment and infection control measures.
From a global health perspective, the rise of Candida auris has become symbolic of a larger problem: we’re entering an era where antimicrobial resistance isn’t just about superbugs like MRSA or drug-resistant tuberculosis. Fungi are joining the fight—and in many ways, they are stealthier adversaries.
But how did Candida auris become so widespread in such a short period? Is climate change playing a role? Could our use of antifungals in agriculture and medicine be accelerating this resistance? And perhaps most importantly: what can we do to stay ahead of this fungal foe?
These are the questions we’ll explore throughout this series. Because understanding Candida auris—and the challenges it presents—is not just a matter for microbiologists or infection control experts. It’s an issue that touches everyone, from hospital staff to patients, public health workers to policymakers.
Epidemiology
Global Distribution and Affected Regions
How does a microscopic fungus manage to become a worldwide problem in just over a decade?
Since its discovery in 2009, Candida auris has made an astonishing journey across the globe. Outbreaks have been documented in countries as diverse as the United States, the United Kingdom, India, South Africa, Venezuela, Spain, and Kenya. And this isn’t a case of isolated infections popping up here and there. We’re talking about healthcare-associated outbreaks that have sometimes shut down entire hospital units and prompted emergency public health responses.
The Centers for Disease Control and Prevention (CDC) has labeled C. auris as an urgent threat, and for good reason. In the U.S. alone, clinical cases tripled between 2019 and 2021, with 2022 seeing even more aggressive spread across multiple states. Europe has seen similar concerns, with outbreaks reported in long-term care facilities in Spain and Italy.
Interestingly, C. auris didn’t spread from a single origin. Genomic analysis has revealed four to five distinct clades of the fungus, emerging independently across different parts of the world—an unusual and concerning phenomenon. Why did this happen? Some researchers speculate that climate change may be playing a role, enabling environmental fungi to adapt to higher body temperatures and thus become pathogenic to humans.
Others suggest the widespread use of antifungal agents—particularly azoles in agriculture and healthcare—might have selected for resistant strains. Regardless of the cause, C. auris has arrived, and it’s not going away quietly.
Populations at Risk
So, who’s most at risk of getting infected with Candida auris?
While healthy people are unlikely to develop C. auris infections, those with weakened immune systems are highly vulnerable. This includes:
- Hospitalized patients, especially those in intensive care units (ICUs)
- Elderly individuals in long-term care facilities
- Patients with invasive devices such as central lines, feeding tubes, or ventilators
- People with chronic conditions like diabetes, kidney disease, or cancer
- Recent surgical patients or those receiving broad-spectrum antibiotics or antifungals
It’s important to note that C. auris doesn’t always cause illness. In many cases, individuals become colonized, meaning the fungus lives on their skin or in their bodies without causing symptoms. But colonized patients can still transmit the fungus to others and may eventually develop infections if their health deteriorates.
There’s also a growing concern about asymptomatic transmission. How many people might be carrying the fungus without even knowing it? And how often does colonization lead to serious illness? These are still open questions, and they highlight just how much we still need to learn about C. auris.
One of the biggest challenges in managing outbreaks is the silent spread within healthcare systems. A patient may test positive weeks after admission, but by then, the fungus may already have spread to others or contaminated surfaces and equipment. It’s like trying to chase smoke in the wind—you know it’s there, but it’s maddeningly hard to catch.
And here’s the unsettling part: as our population ages and more people require long-term care or intensive treatments, the number of individuals vulnerable to C. auris is only expected to grow.
So what does this mean for the future of infection control and hospital safety? Can we protect high-risk populations without disrupting healthcare delivery? And are we prepared to manage a fungus that doesn’t play by the old microbial rulebook?
These are the questions that will shape the next phase of our response to this emerging threat.
Pathogenesis
Mechanisms of Infection and Virulence Factors
What makes Candida auris so successful as a pathogen? And why is it more dangerous than its fungal cousins?
Fungi are often underestimated in the world of infectious diseases, but Candida auris has made it clear that these organisms deserve serious attention. Unlike some other fungi that rely on weakened immune systems to get a foothold, C. auris seems to have a toolkit specifically designed for survival and spread—especially in hospital environments.
Let’s unpack that toolkit.
First, C. auris is incredibly adhesive. It can stick to human skin, plastic surfaces, and medical devices with remarkable tenacity. Once it latches on, it can form biofilms—communities of microorganisms encased in a slimy matrix that protects them from antifungal agents and immune system attacks. Think of biofilms as microbial bunkers: they make treatment harder and persistence easier.
Second, C. auris thrives in warm, moist environments, which makes the human body an ideal host—and hospital settings, with all their equipment, warm rooms, and constant patient turnover, a convenient playground. Unlike some other Candida species that prefer specific niches, C. auris appears to be more adaptable. It can colonize the skin, wounds, ears, and even respiratory tracts.
Another part of its survival strategy is immune evasion. While not as well-studied as bacteria or viruses in this regard, C. auris seems to trigger a muted immune response, allowing it to slip under the radar and establish infection before the body mounts an effective defense.
And perhaps most alarmingly: this fungus is remarkably good at surviving hostile conditions. It’s been shown to tolerate high temperatures, salt concentrations, and disinfectants that would kill off many other microbes. It’s not just sneaky—it’s tough.
So, why is this all so important? Because understanding how C. auris infects and persists is crucial for figuring out how to stop it. If it hides in biofilms, we need agents that can break through that armor. If it evades immune responses, we need to learn how to boost host defenses. And if it thrives on surfaces, then infection control needs to be more rigorous than ever before.
Resistance to Antifungal Treatments
Now comes the elephant in the room: antifungal resistance. What happens when the drugs we rely on to treat infections simply don’t work?
C. auris is often resistant to three major classes of antifungal drugs:
- Azoles, such as fluconazole, which target the fungal cell membrane
- Polyenes, like amphotericin B, a powerful but toxic option
- Echinocandins, which inhibit fungal cell wall synthesis and are often used as last-resort treatments
Some C. auris isolates are resistant to all three classes, creating what’s known as pan-resistant strains. In these cases, there may be no effective antifungal treatment available. That’s a chilling prospect, especially for patients with bloodstream infections, where timely and effective therapy is critical.
But how did this resistance come about?
It’s likely a combination of factors: overuse of antifungal medications, use of antifungals in agriculture, environmental pressures, and—importantly—the inherent genetic adaptability of C. auris. This fungus seems to evolve resistance more quickly than its relatives, and it’s able to pass resistance traits along rapidly.
Even when antifungals are technically “effective” in laboratory tests, clinical outcomes don’t always match. Why? Biofilms, drug penetration issues, or strain-specific differences may all play a role.
And here’s a question researchers are still trying to answer: are we seeing resistance because C. auris is being exposed to antifungals too often—or is it just naturally good at developing resistance on its own?
Either way, the implications are serious. The rise of drug-resistant fungi mirrors the more familiar story of antibiotic resistance in bacteria. But while we’ve spent decades building strategies to address resistant bacteria, we’re still playing catch-up when it comes to fungi.
Can we outpace the resistance? Can new antifungal drugs keep up with C. auris‘s ability to adapt? And what happens if they can’t?
The pathogenesis of Candida auris is a warning—a signal that we need better diagnostics, more effective treatments, and a deeper understanding of how fungi behave in the human body.
Clinical Manifestations
Common Signs and Symptoms
So, what does a Candida auris infection actually look like in a real-world clinical setting? What clues should a healthcare provider be watching for?
Unfortunately, C. auris doesn’t make it easy. Unlike more recognizable infections—say, chickenpox with its telltale rash or influenza with its classic fever-cough-fatigue triad—C. auris often presents without any distinguishing clinical features. That’s part of what makes it so dangerous.
In the majority of cases, C. auris manifests as candidemia, or a bloodstream infection. Patients may experience fever and chills, but here’s the twist: these symptoms frequently persist despite broad-spectrum antibiotic treatment. This can be a subtle but critical red flag, especially in patients with central lines or recent surgeries. When the usual antibiotics fail, clinicians should begin to suspect fungal involvement—especially in high-risk environments like ICUs or long-term care facilities.
But Candida auris isn’t confined to the bloodstream. In some patients, particularly those who are colonized, the fungus can seed other body sites. For example, wound infections can develop in surgical sites or pressure ulcers, leading to delayed healing, localized inflammation, and tissue necrosis. In catheterized patients, C. auris may cause urinary tract infections that resist standard treatments, presenting as cloudy urine, discomfort, or even systemic symptoms. And yes, the ears are still relevant—C. auris was first isolated from ear discharge, and though rare, otitis caused by the fungus can still occur.
Respiratory tract infections are less common, but they have been reported. In ventilated patients, particularly those already colonized, C. auris may be isolated from tracheal aspirates, though it’s sometimes difficult to determine whether it represents true infection or colonization.
The underlying problem is that C. auris infections are non-specific in presentation and closely mimic bacterial infections. When someone is already critically ill, the signs of a fungal infection may simply be lost in the noise. Fever, low blood pressure, increased heart rate—these could be sepsis from almost any cause. So, when should we suspect Candida auris?
That question doesn’t always have a clear answer. But one pattern is becoming clear: C. auris often surfaces in patients who aren’t getting better on antibiotics, especially when they’ve had recent surgeries, invasive devices, or long hospital stays. In such cases, it’s essential to broaden the diagnostic lens and consider fungal pathogens, even when they aren’t the usual suspects.
Complications Associated with Infections
Once Candida auris establishes an infection, what happens next? And why are the outcomes often so poor?
The complications of C. auris are not just medical—they’re systemic. Let’s begin with the most immediate: disseminated infection. Once the fungus reaches the bloodstream, it can rapidly spread to multiple organs. This can lead to septic shock, multi-organ failure, and death. The mortality rate from invasive C. auris infections has been reported to range from 30% to over 60%, especially when diagnosis is delayed or appropriate antifungal therapy isn’t immediately available.
Even when the infection is caught early, treatment is not always straightforward. Resistance to multiple antifungal drugs can complicate management and prolong the course of illness. In some cases, even with therapy, patients remain chronically colonized, which poses an ongoing risk both for themselves and for others around them.
Colonization itself may not cause symptoms, but it has serious implications. A colonized patient can unknowingly become a source of transmission within a facility, contaminating bedding, furniture, and even healthcare workers’ clothing. If the patient’s condition later deteriorates—say, due to a new surgery or immune suppression—C. auris can strike again, this time as a full-blown infection.
Then there’s the long-term picture. Patients who survive severe C. auris infections may face prolonged hospitalization, repeated courses of antifungal therapy, and even long-term complications like kidney damage or neurological deficits, depending on the organs affected. Moreover, these patients often require isolation and specialized care, which can limit access to rehabilitation services and prolong recovery.
And what about the healthcare system? An outbreak of C. auris can strain infection control resources, necessitate deep cleaning of rooms and equipment, and sometimes even force the temporary closure of hospital wards. This isn’t just a burden on infection prevention teams—it affects admissions, surgical schedules, and even public trust in hospital safety.
So we must ask: how do we recognize and respond to a pathogen that hides behind familiar symptoms, resists treatment, and leaves a long shadow even after it’s gone? What protocols can help us intervene before it spreads—or before it’s too late?
The answers lie in the next sections, where diagnosis and treatment strategies take center stage.
Diagnosis
Laboratory Methods for Accurate Identification
So, if Candida auris doesn’t present with clear, unique symptoms, how do we know when we’re dealing with it? More importantly — how do we know for sure?
The answer, unfortunately, isn’t simple. Diagnosing Candida auris requires more than clinical suspicion; it demands precision in the lab — and even then, there’s room for error.
Standard diagnostic methods, such as culture-based identification, are often the first step. Clinical samples — blood, urine, wound swabs, or respiratory secretions — are cultured on fungal media. If colonies of Candida are found, they’re typically passed through automated identification systems like VITEK, API 20C AUX, or BD Phoenix. And this is where things start to get tricky.
Many of these conventional systems are not equipped to correctly distinguish C. auris from closely related species. Instead, they often misidentify it as Candida haemulonii, Candida sake, or even Rhodotorula. Why? Because C. auris is relatively new to the scene, and older databases simply don’t include it — or if they do, they lack the genetic resolution to tell the species apart.
So, what’s the workaround? How do clinicians make the right call?
To confirm C. auris definitively, we now rely on advanced diagnostic tools, such as MALDI-TOF mass spectrometry— a sophisticated technique that matches the unique protein fingerprint of a microbe against a curated database. When the database includes C. auris (which, to be clear, it didn’t until relatively recently), the identification is fast and accurate.
Alternatively, molecular methods such as PCR (polymerase chain reaction) or real-time nucleic acid amplification tests can directly detect C. auris DNA from patient samples or environmental swabs. These are increasingly being deployed in hospitals during outbreaks, because they allow for quicker identification and faster containment.
And let’s not overlook whole genome sequencing — the gold standard for confirming the identity of C. auris and for tracing the clade or strain involved in a specific outbreak. This level of detail helps us understand transmission patterns, resistance profiles, and even evolutionary changes. But sequencing is time-consuming, expensive, and not available in most frontline healthcare settings.
Which raises a practical question: Are our current diagnostic systems good enough? Can small or under-resourced hospitals detect C. auris in time to act? Or are we missing cases simply because we don’t have the right tools?
Challenges in Distinguishing from Other Candida Species
It’s important to emphasize that Candida auris is part of a larger family — the Candida genus — which includes many other species that can cause disease in humans. But C. auris behaves differently, spreads differently, and responds to treatment differently. That makes misidentification not just a laboratory error — but a clinical hazard.
Let’s say a patient’s blood culture comes back positive for what looks like Candida haemulonii. Based on that result, the medical team might assume typical antifungal susceptibility and start fluconazole. But what if that organism is actually C. auris, which is often resistant to fluconazole? That delay in adjusting therapy could cost the patient their life.
And it’s not just about treatment choices. Misidentification means missed opportunities for infection control. If a colonized or infected patient isn’t recognized as a carrier of C. auris, they won’t be isolated, their room won’t be cleaned with enhanced disinfectants, and contact tracing won’t be initiated. That’s how outbreaks begin — quietly, and often invisibly.
There’s also a deeper, more systemic issue. Because C. auris hides behind familiar names and resembles common commensals under the microscope, it often flies under the radar. How many patients have died from “unknown fungal infections” that were actually C. auris? How many outbreaks have gone unreported because no one realized what they were dealing with?
Fortunately, awareness is growing. Newer identification platforms now come pre-loaded with C. auris profiles. Diagnostic labs are being trained to flag suspicious isolates. Public health labs are offering confirmatory testing. But the lag time between detection and response still varies widely between countries — and even between hospitals in the same city.
So the big question remains: can we scale up accurate, rapid Candida auris diagnostics across the board? Or will this fungus continue to exploit gaps in our lab infrastructure?
The diagnostic challenge is at the heart of the C. auris problem. Without fast and reliable identification, everything else — treatment, containment, prevention — is delayed. And in the world of infectious disease, delays are often deadly.
Treatment
Current Antifungal Therapies and Their Effectiveness
Once Candida auris is identified, the next urgent question is obvious: what can we use to treat it?
Unlike many other fungal infections, the treatment landscape for C. auris is frustratingly narrow. This isn’t because we haven’t developed antifungal drugs — we have several classes — but because C. auris has already shown an unsettling ability to resist them. In some cases, it resists all of them.
Typically, the first-line treatment for C. auris infections is an echinocandin, such as micafungin, anidulafungin, or caspofungin. These drugs inhibit the synthesis of a key component of the fungal cell wall, and they’re generally effective against most C. auris strains — at least for now. However, resistance to echinocandins is increasingly being reported, especially in regions where the drug is used extensively or misused.
When echinocandins don’t work — or if the patient isn’t improving — clinicians may escalate to lipid-formulated amphotericin B, a more potent but also more toxic option. Amphotericin B acts by disrupting the fungal cell membrane, but its use is often limited by side effects such as kidney damage, electrolyte disturbances, and infusion-related reactions. In other words, it works — but it comes at a cost.
There’s also fluconazole, a commonly used azole antifungal. But here’s the catch: most C. auris isolates are intrinsically resistant to fluconazole, making it unreliable as a treatment option. Other azoles like voriconazole or posaconazole might show better activity in some cases, but susceptibility testing is essential — and it takes time.
What happens when the organism is resistant to all three major classes — echinocandins, azoles, and amphotericin B? These are referred to as pan-resistant strains, and they represent the nightmare scenario: patients for whom no standard antifungal treatment is expected to work.
This leads to difficult, ethically complex questions. Should doctors attempt combination therapies, even if there’s no clinical data to support them? Should they try unapproved or experimental treatments? What does informed consent look like in these high-stakes, last-resort scenarios?
In such cases, treatment becomes less about following evidence-based guidelines and more about strategic improvisation, guided by whatever microbiological data are available, the patient’s condition, and sheer clinical intuition.
And that’s assuming the drugs are even available. In many parts of the world, echinocandins are prohibitively expensive or not stocked at all. Amphotericin B may be available in its older, more toxic form but not in the safer lipid formulations. In these settings, even if the diagnosis is correct, the tools to fight back may simply not be there.
Emerging Treatment Options and Research
So where does that leave us? Are we doomed to be outpaced by Candida auris’s resistance evolution, or is there hope on the horizon?
Fortunately, the answer is: there is hope — but it’s still emerging.
One of the most promising new antifungal agents is ibrexafungerp, a glucan synthase inhibitor that acts similarly to echinocandins but with a different molecular structure. Early studies have shown that it retains activity against some echinocandin-resistant C. auris isolates, which makes it a potential game-changer for difficult cases.
Another compound in development is fosmanogepix, a novel agent that inhibits a fungal enzyme involved in cell wall assembly. It has shown broad-spectrum activity, including against multidrug-resistant fungi like C. auris. If clinical trials go well, this drug could provide a much-needed new mechanism of action.
Researchers are also exploring the use of combination therapies — for example, echinocandins plus azoles, or echinocandins plus amphotericin B — to enhance efficacy or prevent the emergence of resistance. These approaches are still largely experimental, but in life-threatening infections, they may be considered on a compassionate-use basis.
Immunotherapy is another frontier. Scientists are investigating whether monoclonal antibodies or immune modulatorscould help the body better recognize and fight Candida auris, especially in immunocompromised individuals. It’s early-stage research, but given how stealthy C. auris is, empowering the immune system might be just as important as targeting the fungus itself.
And let’s not forget the push for rapid antifungal susceptibility testing. Knowing within hours — rather than days — whether a strain is resistant could help guide therapy more accurately, reduce unnecessary exposure to ineffective drugs, and improve outcomes. Advances in this space could be just as crucial as new drugs themselves.
So, the big questions loom: Will these emerging treatments arrive in time to make a difference? Can they be manufactured affordably and distributed globally? And will they work against the inevitable next strain that C. auris throws at us?
In the fight against this adaptive, persistent fungus, we’ll need more than just new drugs. We’ll need a flexible, well-resourced healthcare system capable of deploying those drugs quickly, accurately, and equitably — wherever the need arises.
Infection Control and Prevention
Strategies to Prevent Spread in Healthcare Facilities
If Candida auris is so good at hiding, resisting treatment, and spreading quietly through healthcare systems — how do we stop it?
That question has become the driving concern for infection control specialists around the world. Unlike most pathogens that are neutralized by standard cleaning protocols and hand hygiene, C. auris demands a far more aggressive response. It isn’t just about patient care anymore — it’s about institutional defense.
The cornerstone of C. auris prevention is early identification and isolation. As soon as a patient is confirmed or even suspected to carry C. auris, they must be placed under strict contact precautions. This includes assigning them a single room, preferably with dedicated equipment that won’t be shared with other patients — stethoscopes, thermometers, even blood pressure cuffs. In outbreak scenarios, cohorting infected patients in a designated unit or wing may be necessary to prevent hospital-wide spread.
But identification is only part of the puzzle. The second line of defense is the environment — and this is where C. aurisbreaks the rules. Unlike other Candida species that primarily inhabit mucosal surfaces, C. auris is a skin colonizer and a surface survivor. It can persist on bed rails, curtains, floors, IV poles, and even mobile workstations for days or weeks, resisting many commonly used disinfectants.
This means that typical hospital cleaning protocols — the ones designed for bacteria like MRSA or Clostridioides difficile— may not be enough. Facilities must deploy EPA-registered hospital-grade disinfectants that are proven effective against C. auris, often containing chlorine-based compounds or hydrogen peroxide vapor. Terminal cleaning (deep cleaning after patient discharge) must be thorough, monitored, and repeated as necessary.
Hand hygiene remains critical, but even this has its caveats. Alcohol-based hand sanitizers, while effective against many microbes, may be less reliable for eradicating C. auris from contaminated hands — especially if glove use is inconsistent. Soap and water followed by proper drying and careful glove removal is emphasized in high-risk units.
There’s also an important human element to all of this: staff awareness. Nurses, physicians, environmental services staff — everyone needs to know what C. auris is, how it spreads, and what measures are non-negotiable. Training isn’t just about rules; it’s about mindset. Do staff recognize the risk when a patient is flagged for multidrug-resistant Candida? Do they understand why this isn’t just another fungal infection?
And what about visitors? Should families wear gowns and gloves? How do we balance infection control with emotional support and compassionate care?
Every hospital must wrestle with these questions in real-time, often while juggling limited resources and ongoing outbreaks of other pathogens. But what’s clear is this: when it comes to Candida auris, proactive containment is far easier than reactive crisis management.
Guidelines for Hygiene and Sanitation
If you were designing a hospital from scratch to resist C. auris, what would you prioritize? The answers are surprisingly straightforward — and frustratingly difficult to implement universally.
First, every healthcare facility must have a standardized protocol for managing multidrug-resistant organisms (MDROs), and that protocol needs to include C. auris specifically. It’s not enough to lump it into the general “fungal infections” category. This fungus behaves differently and requires its own playbook.
The CDC and WHO have both issued guidelines for C. auris control. Key recommendations include:
- Rigorous environmental disinfection using sporicidal agents
- Daily and terminal cleaning of patient rooms with agents known to kill C. auris
- Screening of close contacts and high-risk patients (e.g., transfers from affected facilities)
- Cohorting of staff and equipment where feasible
- Clear signage indicating contact precautions
But real-world implementation is not always so seamless. In busy hospitals, disinfectants may be diluted improperly, high-touch areas may be missed during cleaning rounds, and staff turnover can lead to lapses in training. Plus, the added cost of C. auris-specific precautions — in both time and materials — isn’t always reimbursed or supported at the administrative level.
Let’s not forget long-term care facilities. These environments, often less equipped than acute hospitals, are uniquely vulnerable. They house patients for extended periods, often with chronic wounds, catheters, and tracheostomies — all of which increase colonization risk. Yet these facilities may lack the staffing, training, or financial support to implement full-scale C. auris protocols. Should infection control funding be restructured to prioritize such high-risk settings?
And what about outside the hospital? Can C. auris survive on public transit? Are there risks to caregivers at home? While community transmission appears limited, it’s not impossible. The fungus has been detected on the skin and clothing of asymptomatic carriers — people who look and feel perfectly healthy but could be unwitting vectors in the chain of transmission.
As our understanding grows, guidelines will continue to evolve. But one thing is clear already: staying ahead of Candida auris requires a shift in institutional culture, not just cleaning products or policy memos. It requires vigilance, transparency, cross-disciplinary collaboration — and above all, urgency.
So we have to ask: Are we training our healthcare workers fast enough? Are we giving them the tools and support they need? And can we afford to keep underestimating a fungus that refuses to play by the rules?
Recent Developments (2025–2026)
Notable Outbreaks and Case Studies
What’s happened lately with Candida auris? Has the situation improved — or are we seeing new challenges emerge?
The years 2025 and 2026 have been pivotal in the ongoing story of Candida auris. Despite widespread awareness and more robust infection control practices in many high-income countries, C. auris continues to thrive in unexpected ways — often adapting faster than healthcare systems can respond.
One of the most striking developments has been a series of simultaneous outbreaks in geographically distant regions, suggesting not only that C. auris is endemic in many healthcare networks but that its routes of transmission may be more complex than we thought. For instance, outbreaks reported in 2025 in parts of Southeast Asia and Central Europe involved pan-resistant strains — organisms with no susceptibility to any of the three major antifungal classes. These events triggered temporary ICU closures, emergency response coordination, and in some cases, international reporting under WHO’s revised IHR (International Health Regulations) protocols.
In the United States, a particularly notable event unfolded in late 2025 when a long-term acute care hospital in the Midwest experienced a sustained C. auris outbreak despite adherence to CDC-recommended protocols. Genomic analysis revealed a new clade of C. auris with subtle mutations in cell wall structure — enough to reduce disinfectant efficacy and escape traditional PCR primers used for detection. This case has fueled new discussions about the plasticity of the organism’s genome and how evolutionary adaptation may be accelerating under the selective pressures of high-intensity infection control.
Meanwhile, in South America, a multi-center case study published in early 2026 described a C. auris outbreak that began with a single colonized patient transferred between facilities. Within six weeks, over 30 individuals were affected across four hospitals. This scenario highlighted the critical role of patient transfer networks — and how lapses in inter-facility communication can turn colonization into crisis almost overnight.
Are these outbreaks failures of policy or reflections of the organism’s resilience? What would it take to build truly “fungus-proof” healthcare systems? And how many more near-misses will it take before we begin treating fungal threats with the same seriousness as viral and bacterial epidemics?
Advances in Diagnostic Tools and Treatments
Thankfully, it hasn’t been all bad news.
In parallel with these outbreaks, several exciting advances in Candida auris management have emerged. One of the most promising developments has been the commercial availability of next-generation PCR assays that can detect C. aurisdirectly from skin swabs in under two hours — a major improvement over older systems that took days and required pure culture growth. These rapid diagnostics are now being rolled out in high-risk facilities, particularly in ICUs and long-term care settings, where early detection is most critical.
A breakthrough came in mid-2026 when the FDA granted emergency use authorization for a novel antifungal compound: MYC-113, a first-in-class agent targeting fungal lipid metabolism. Early trials show that it is effective against most known resistant strains of C. auris, and significantly, it appears to work synergistically with echinocandins, opening the door to combination therapy regimens that are both potent and less toxic.
At the same time, global health agencies have begun investing in real-time fungal surveillance systems, powered by AI-driven analytics. These platforms can detect unusual resistance trends across hospital networks, helping to anticipate outbreaks before they spiral. Some are even integrating environmental swab data and colonization screenings from patient intake — a step toward true predictive infection control.
There’s also growing momentum behind fungal vaccine research — long a neglected area. While no vaccine for Candida auris exists yet, early-stage trials using mRNA platforms (inspired by COVID-19 vaccine success) are underway. These vaccines aim to prime the immune system against a broad set of fungal surface proteins, potentially offering cross-protection against multiple Candida species.
But with every advancement come new questions. Will hospitals in low- and middle-income countries be able to afford and implement these technologies? Will emerging strains continue to outpace our innovations? And how can regulatory systems be streamlined to ensure new treatments reach patients before resistance makes them obsolete?
The 2025–2026 period has been both a warning and a window — a stark reminder that Candida auris is not standing still. It’s evolving, spreading, and learning from us as quickly as we learn about it. Whether we rise to meet that challenge will depend not just on science, but on systems — of policy, communication, training, and international cooperation.
Frequently Asked Questions (FAQ)
1. What exactly is Candida auris and why is it causing such concern?
Candida auris is a species of fungus that has emerged globally as a serious public health threat due to its ability to cause invasive infections, spread rapidly in healthcare settings, and resist multiple antifungal drugs. Its stealthy nature, high mortality rate in vulnerable patients, and persistence on surfaces make it especially dangerous.
2. Where did Candida auris come from, and how did it spread so quickly?
The fungus was first identified in Japan in 2009, but retrospective studies suggest it may have been around even earlier. Genomic studies show that it likely emerged independently in different parts of the world — a phenomenon still not fully understood. Its spread has been facilitated by global travel, healthcare transfers, and inadequate infection control protocols in some settings.
3. Who is at the highest risk of getting infected with C. auris?
Patients who are already hospitalized, especially those in intensive care units, are most at risk. This includes individuals with central lines, ventilators, or recent surgeries, as well as those who are immunocompromised or have chronic illnesses like diabetes or kidney disease.
4. How is C. auris different from other Candida species?
Unlike most Candida species, C. auris often colonizes the skin, survives for extended periods on surfaces, and shows high levels of antifungal resistance. It also spreads more easily between patients and is notoriously difficult to identify with standard lab techniques.
5. What symptoms should raise suspicion for Candida auris?
Symptoms are often non-specific, typically involving fever and sepsis that don’t respond to antibiotics. Since C. auris can infect multiple body systems — blood, wounds, urinary tract, even the respiratory tract — any unexplained or treatment-resistant infection in a high-risk patient should prompt investigation.
6. Why is diagnosing C. auris so challenging?
Many laboratory systems still misidentify it as other fungi, and the symptoms overlap with bacterial and other fungal infections. Rapid, species-specific diagnostic tools are becoming more available, but access to them varies widely by region and institution.
7. What treatments are currently effective against C. auris?
Echinocandins are usually the first-line treatment, but some strains are resistant. Amphotericin B may be used in resistant cases, although it carries significant side effects. Newer drugs like ibrexafungerp and experimental agents such as MYC-113 are showing promise, especially against multidrug-resistant strains.
8. Can Candida auris be prevented?
Yes, but it requires rigorous infection control. This includes early detection, isolation of infected patients, meticulous environmental cleaning, and staff education. Prevention efforts also hinge on proper communication between facilities during patient transfers.
9. Is C. auris only a problem in hospitals?
Primarily, yes — most outbreaks and cases have occurred in healthcare settings. However, colonized individuals may carry it into other environments, and there is a growing concern about its persistence in long-term care and assisted-living facilities.
10. Is a vaccine for Candida auris being developed?
There is no approved vaccine yet, but research is underway. Some experimental approaches, including mRNA-based platforms, are in the early stages of testing and may offer protection in the future, especially for high-risk groups.
Conclusion
Candida auris has evolved from a medical curiosity to a global public health challenge in little more than a decade. What makes it uniquely threatening is not just its resistance to treatment, but its ability to spread silently, mimic other infections, and adapt in real-time to our medical countermeasures.
We’ve seen how it targets the most vulnerable — critically ill patients, long-term care residents, and individuals with weakened immune systems. We’ve learned that it doesn’t play by the usual microbial rules: it clings to surfaces, evades detection, and resists the very drugs we rely on most. And as we’ve seen in recent years, it continues to outmaneuver even well-resourced healthcare systems.
But we’ve also witnessed remarkable advances. From faster diagnostic tools to promising new treatments, and from global surveillance initiatives to stronger hospital protocols, the tide is beginning to turn. The question now is: will our pace of innovation and preparedness keep up with C. auris’s capacity to change?
The story of Candida auris is still unfolding. It’s not just a cautionary tale about fungal resistance — it’s a call to action for a more vigilant, better-resourced, and globally coordinated response to emerging pathogens. If we can rise to this challenge, we’ll not only contain C. auris, but we’ll also be better prepared for the next microbial threat that comes knocking.