Bowel Cancer Tumor Markers
Bowel Cancer Tumor Markers: The Full Guide
- What Are Tumor Markers and How Do They Work?
- Carcinoembryonic Antigen (CEA): The Primary Marker for Bowel Cancer
- Other Emerging and Supporting Tumor Markers
- Role of Tumor Markers in Detecting Recurrence
- Limitations and Accuracy of Tumor Marker Testing
- How Tumor Markers Are Collected and Analyzed
- Tumor Markers and Personalized Medicine in Bowel Cancer
- Comparing Tumor Markers Across Bowel Cancer Stages
- Role of Tumor Markers in Monitoring Cancer Recurrence
- Emerging Markers: ctDNA and Liquid Biopsy
- How Tumor Markers Guide Chemotherapy and Treatment Response
- Interpreting Results: What Numbers Really Mean
- Tumor Markers and Genetic Mutations in Bowel Cancer
- Comparing Tumor Markers to Imaging and Colonoscopy
- Limitations and Pitfalls of Tumor Marker Testing
- Future of Tumor Marker Development
- Tumor Markers and Post-Treatment Surveillance
- Integration with Recurrence Risk Stratification
- Real-World Examples of Tumor Marker Use
- Global Guidelines on Tumor Marker Use in Bowel Cancer
- Limitations and False Positives of Tumor Markers
- Tumor Markers and Personalized Medicine
- Comparing Bowel Cancer Markers with Other Conditions
- Patient Tips: Understanding and Using Tumor Marker Tests
- Limitations of Tumor Markers in Bowel Cancer Diagnosis
- How Tumor Markers Are Used in Treatment Monitoring
- Emerging Tumor Markers and Molecular Biomarkers
- Integrating Tumor Marker Testing into Personalized Care
- FAQ: Bowel Cancer Tumor Markers
What Are Tumor Markers and How Do They Work?
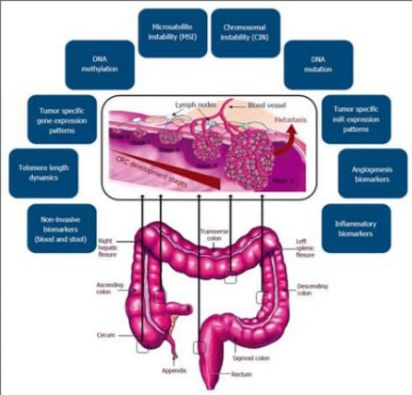
Tumor markers are substances—usually proteins or molecules—produced by cancer cells or by the body in response to cancer. These markers can be found in blood, urine, or tissue samples and serve as valuable indicators for diagnosing and tracking cancers, including bowel cancer.
In the context of bowel (colorectal) cancer, tumor markers are especially useful for:
- Monitoring response to treatment
- Detecting recurrence after surgery or therapy
- Providing insight into cancer progression
However, tumor markers are not used alone to diagnose cancer, as many can also be elevated in non-cancerous conditions. Their value lies in being part of a broader diagnostic and monitoring strategy.
Carcinoembryonic Antigen (CEA): The Primary Marker for Bowel Cancer
CEA is the most commonly used tumor marker for colorectal cancer. It is a glycoprotein involved in cell adhesion and is typically present at very low levels in healthy adults. Elevated levels may suggest the presence of cancer, particularly in the colon or rectum.
CEA Test Usage:
- Baseline measurement before treatment
- Monitoring recurrence after surgery
- Assessing treatment effectiveness during chemotherapy
CEA levels are measured through a simple blood test. While high levels might indicate a problem, not all bowel cancer patients will have elevated CEA, and other conditions—such as smoking, inflammation, or liver disease—can also affect levels.
| CEA Level Range | Interpretation |
| < 3 ng/mL | Normal for non-smokers |
| 3–5 ng/mL | Normal for smokers |
| > 5 ng/mL | May indicate cancer or other disease |
Although CEA is not perfect, it is still the most frequently used tumor marker in colorectal cancer care.
Other Emerging and Supporting Tumor Markers
Besides CEA, several other tumor markers are being studied and occasionally used in clinical settings:
- CA 19-9: More often associated with pancreatic cancer, but may be elevated in advanced colorectal cases.
- CA 125: Typically linked to ovarian cancer, but can sometimes be present in bowel cancer with peritoneal involvement.
- Circulating Tumor DNA (ctDNA): A newer class of markers, representing small fragments of DNA from cancer cells in the bloodstream.
- Microsatellite Instability (MSI) and Mismatch Repair Proteins (MMR): These are not traditional blood markers but genetic profiles found in tumor tissue that guide treatment.
These markers are increasingly important in personalizing treatment, especially in identifying patients who may benefit from immunotherapy or more aggressive surveillance.
Role of Tumor Markers in Detecting Recurrence
One of the most critical uses of tumor markers in bowel cancer is post-treatment monitoring. After curative surgery or chemotherapy, patients undergo regular blood tests to track markers like CEA.
An upward trend in tumor marker levels—even if still within the normal range—can indicate the possibility of recurrence. In such cases, doctors may order imaging tests like CT scans or PET scans to identify new tumor growth.
Patients who undergo consistent follow-up using tumor markers tend to have earlier detection of recurrence, allowing for potentially curative second surgeries or early second-line treatments. However, false positives can occur, underscoring the need for combined clinical judgment and diagnostic imaging.
Limitations and Accuracy of Tumor Marker Testing
Although tumor markers such as CEA can offer valuable insights, they are not definitive tools for either diagnosing bowel cancer or making treatment decisions on their own. Their interpretation is complex and must be viewed in the broader clinical context.
Key limitations:
- Lack of specificity: Elevated levels may result from non-cancerous conditions such as liver disease, smoking, or inflammatory bowel disease.
- False negatives: Some bowel cancer patients may have normal tumor marker levels throughout their illness.
- Individual variability: Normal values can differ depending on a person’s biology, making universal thresholds difficult.
Doctors rely on trends over time, not just single values. A steady rise in markers like CEA after surgery or chemotherapy might signal recurrence, even if the values are modest.
Therefore, while tumor markers are a useful monitoring tool, they are never a replacement for imaging scans, colonoscopy, or pathology results.
How Tumor Markers Are Collected and Analyzed
The collection process for tumor markers is straightforward and non-invasive in most cases. Here’s how it typically works:
- Blood draw: For markers like CEA, CA 19-9, or ctDNA, a simple venipuncture is performed, often during routine checkups.
- Tissue biopsy: Markers like MSI or MMR require a sample from the tumor, usually taken during a colonoscopy or surgery.
- Analysis in a lab: Specialized laboratories use enzyme-linked immunosorbent assay (ELISA), PCR, or genomic sequencing technologies to evaluate the samples.
| Marker Type | Sample Needed | Typical Use Case |
| CEA | Blood | Monitoring, recurrence detection |
| CA 19-9 | Blood | Supplementary in advanced cases |
| ctDNA | Blood | Residual disease, emerging use case |
| MSI/MMR | Tumor tissue | Prognosis, treatment personalization |
The timing of tests is crucial. For instance, testing too soon after surgery might give inaccurate results due to post-operative inflammation. Most guidelines recommend waiting several weeks after treatment for the first accurate marker reading.
Tumor Markers and Personalized Medicine in Bowel Cancer
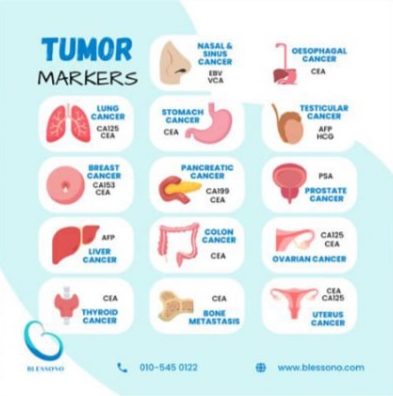
Advances in tumor marker science are rapidly shifting bowel cancer management toward personalized medicine. Instead of one-size-fits-all treatment, tumor markers help guide therapies based on individual tumor behavior.
Examples include:
- MSI-high or MMR-deficient tumors: These patients often respond better to immunotherapy (e.g., checkpoint inhibitors).
- KRAS/NRAS/BRAF mutations: Detected through extended genomic panels, these affect eligibility for certain targeted therapies.
- ctDNA: Helps determine whether microscopic cancer remains after surgery, aiding decisions about adjuvant chemotherapy.
This level of detail improves outcomes by avoiding ineffective treatments and reducing unnecessary side effects. It also informs conversations about prognosis and treatment timelines. This is especially relevant in cases where symptoms overlap with non-cancer conditions, such as bowel leakage a sign of cancer or symptom of bowel cancer—underlining the need for precise molecular tools.
Comparing Tumor Markers Across Bowel Cancer Stages
The use and relevance of tumor markers change depending on how advanced the cancer is. In early stages, they may have limited role in detection but become increasingly important in stage III and IV disease for tracking response and recurrence.
| Bowel Cancer Stage | Typical Use of Tumor Markers |
| Stage I | Rarely used, limited sensitivity |
| Stage II | May assist in determining risk and need for chemo |
| Stage III | Important for monitoring during and after treatment |
| Stage IV | Essential for tracking therapy response |
In advanced stages, markers like CEA and ctDNA are frequently ordered every few months. Fluctuations in these values are used alongside imaging to assess treatment efficacy and inform decisions about continuing or switching therapies.
This staged approach to markers also helps distinguish cancer progression from benign conditions. For example, back ache and bowel cancer can co-occur in advanced disease, and tumor markers may guide whether further investigation is needed.
Role of Tumor Markers in Monitoring Cancer Recurrence
After initial treatment—whether surgery, chemotherapy, or radiation—tumor markers play a vital role in monitoring for recurrence, especially in stages II through IV. The most commonly used is CEA, and its levels are tracked routinely every 3 to 6 months for the first few years after treatment.
Why this matters:
- A rising trend in CEA might indicate microscopic cancer cells are growing again, even before visible on scans.
- Stable or decreasing levels often signal effective control or remission.
- Sudden spikes without symptoms might prompt further imaging or biopsy to confirm or rule out recurrence.
However, fluctuations can also result from benign causes like infection, inflammation, or smoking. Physicians always interpret tumor marker results alongside clinical context and imaging.
This proactive surveillance helps in early detection of relapse, improving outcomes by allowing earlier treatment adjustments.
Emerging Markers: ctDNA and Liquid Biopsy
One of the most promising innovations in oncology is circulating tumor DNA (ctDNA). This marker is detected through a liquid biopsy—a simple blood test that identifies tiny fragments of DNA shed by cancer cells into the bloodstream.
Advantages of ctDNA:
- Highly sensitive for detecting minimal residual disease
- Can predict recurrence earlier than traditional imaging or CEA
- Non-invasive, requiring only a blood sample
While still being integrated into clinical guidelines, ctDNA is already influencing decisions such as:
- Whether to offer chemotherapy after surgery
- How aggressively to treat stage II cancers
- Detecting recurrence months before it appears on scans
Its precision aligns well with personalized medicine, although it does not fully replace standard surveillance tools yet.
How Tumor Markers Guide Chemotherapy and Treatment Response
During chemotherapy or targeted therapy for bowel cancer, tumor markers—especially CEA and CA 19-9—are tracked over time to evaluate whether the treatment is working.
- Declining levels usually indicate the tumor is shrinking or stabilizing.
- Plateaus may signal a need to reassess or switch treatment.
- Rising values, particularly in conjunction with new symptoms, suggest the cancer may be progressing.
This approach allows oncologists to adapt quickly without waiting for physical symptoms to worsen or relying entirely on imaging. In metastatic disease, these markers often become a real-time barometer of treatment success.
For patients juggling chronic symptoms like back ache and bowel cancer, markers can help differentiate between cancer-related changes and musculoskeletal issues, guiding decisions on further testing or pain management.
Interpreting Results: What Numbers Really Mean
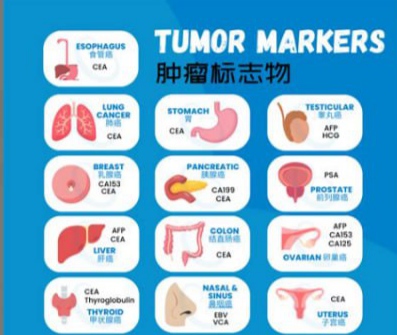
It’s essential for patients to understand that tumor marker levels are not diagnoses—they’re signals. A number alone cannot confirm or rule out bowel cancer, and context is everything.
For example:
- CEA normal range: Typically <5 ng/mL in non-smokers, but smokers may have slightly elevated baseline
- A value of 8 ng/mL in a smoker post-treatment may be considered acceptable if stable
- Rapid increase from 4 to 12 ng/mL over three months may prompt further investigation
Important notes:
- Always consider lab variability—different labs may use different reference ranges
- One-time elevations are less important than trends over multiple tests
- CEA can remain elevated for months after treatment due to inflammation or scarring
Doctors often use charts and graphs to track these trends and communicate results to patients more clearly. Clear communication helps reduce unnecessary fear or false reassurance.
Tumor Markers and Genetic Mutations in Bowel Cancer
Beyond general markers like CEA and CA 19-9, genetic tumor markers are now being integrated into bowel cancer evaluation, especially for advanced or recurrent cases. These include:
- KRAS, NRAS, and BRAF mutations: Common in metastatic colorectal cancer. Knowing whether these mutations are present can guide targeted therapies like cetuximab or panitumumab.
- MSI (Microsatellite Instability): Indicates how well tumor cells repair DNA damage. High MSI often responds better to immunotherapy.
- HER2 amplification: Although rare, it opens options for HER2-targeted therapies, previously used mainly in breast cancer.
Testing for these markers is typically done on a tumor tissue sample, especially after surgery or biopsy. While they’re not used to detect cancer initially, they are essential for personalized treatment planning and predicting therapy response.
These genetic insights reflect the broader shift toward precision oncology—treating not just the cancer location but its unique biological makeup.
Comparing Tumor Markers to Imaging and Colonoscopy
Tumor markers are complementary tools—they do not replace imaging scans or colonoscopy but provide different information.
| Diagnostic Tool | Strengths | Limitations |
| Tumor Markers (e.g. CEA) | Detect biochemical changes, easy to repeat | Not specific, false positives possible |
| Imaging (CT, MRI) | Shows tumor size and location | May miss early recurrence or microscopic spread |
| Colonoscopy | Direct visualization and biopsy of bowel | Limited to bowel lumen, invasive |
When used together, they provide a multidimensional view:
- Imaging shows structure
- Markers show activity
- Colonoscopy confirms surface tumors
This approach is especially useful when evaluating vague issues like whether bowel leakage is a sign of cancer or just post-surgical irritation—markers help determine if further investigation is necessary.
Limitations and Pitfalls of Tumor Marker Testing
Despite their utility, tumor markers come with several limitations that must be carefully navigated:
- Lack of specificity: CEA can be elevated in benign conditions like ulcerative colitis, pancreatitis, or liver disease.
- Not useful for screening: Elevated markers alone do not justify a cancer diagnosis without imaging or pathology.
- False negatives: Some patients with confirmed bowel cancer have normal tumor marker levels, especially in early stages.
- Interference from smoking: CEA levels are chronically elevated in smokers, complicating interpretation.
Patients and even some non-oncology providers can misunderstand elevated markers as proof of active disease, leading to unnecessary anxiety or treatment. Education and a structured approach to interpreting trends rather than single values are crucial.
Future of Tumor Marker Development
The field of tumor markers is rapidly evolving, driven by advances in molecular biology and bioinformatics. Researchers are working on:
- Multi-analyte tests that combine DNA, protein, and RNA data
- AI-assisted marker interpretation, helping oncologists predict recurrence patterns
- Saliva and stool-based biomarker tests for easier monitoring
- Immune system markers (e.g., T-cell signatures) that signal early response to immunotherapy
In the future, routine blood work might detect not only tumor burden but also predict how a person will respond to certain treatments. For those experiencing symptom of bowel cancer but with normal imaging, these next-gen markers could offer new diagnostic pathways.
These innovations aim to individualize care, reduce delays in detection, and avoid unnecessary treatment when tumors are inactive or absent.
Tumor Markers and Post-Treatment Surveillance
After initial treatment, monitoring tumor markers becomes part of routine follow-up for bowel cancer patients. The most commonly used marker is CEA (Carcinoembryonic Antigen). If levels were elevated before treatment and then normalized afterward, it provides a reference point for detecting recurrence.
Regular CEA testing is often scheduled every 3 to 6 months for the first few years. A rising trend—even within the normal range—may signal a recurrence before imaging can detect it. However, isolated fluctuations should be interpreted cautiously. A steady upward trend over multiple tests is more concerning than a single outlier.
Importantly, post-treatment marker testing is not useful for all patients, especially if their cancer never caused an elevated marker level. In such cases, clinicians rely more heavily on colonoscopies and scans.
This approach helps detect recurrence early enough for curative re-intervention in many cases, especially in stage II or III patients.
Integration with Recurrence Risk Stratification
Tumor markers are now being included in risk stratification models, which help determine how closely a patient needs to be monitored after treatment. These models consider:
- Tumor stage at diagnosis
- Lymph node involvement
- Margins after surgery
- Marker dynamics post-treatment
For example, a patient with stage III cancer, positive nodes, and slow-normalizing CEA after surgery might be followed more intensively than a stage I patient with consistently normal CEA.
Tumor markers help oncologists decide:
- Frequency of follow-up appointments
- Imaging intervals
- Timing of re-treatment
For patients reporting vague complaints like back ache and bowel cancer suspicion, these models can help assess if symptoms warrant urgent testing or are likely unrelated.
Real-World Examples of Tumor Marker Use
Let’s look at two hypothetical cases that illustrate how tumor markers influence decisions:
Case 1:
- 62-year-old male, Stage III colon cancer
- Pre-surgery CEA: 15.2 ng/mL
- Post-surgery: 2.1 ng/mL
- One year later: CEA rising to 4.5, then 6.0
Even though still below the abnormal threshold, the upward trend prompts a PET-CT, revealing a small metastatic deposit in the liver. Early resection is performed.
Case 2:
- 70-year-old woman, Stage II rectal cancer
- CEA never elevated
- Complains of fatigue and mild pelvic discomfort
- CEA normal, imaging unremarkable
Despite symptoms, no recurrence is found. The normal marker helped avoid unnecessary invasive tests.
These cases show how tumor markers are part of a larger clinical puzzle—not definitive answers, but highly informative clues.
Global Guidelines on Tumor Marker Use in Bowel Cancer
International medical societies provide structured recommendations for tumor marker use. Here’s a summary comparison:
| Organization | Marker Focused | Use for Monitoring | Use for Screening | Notes |
| ASCO (USA) | CEA | Yes | No | Emphasizes trend over threshold |
| NICE (UK) | CEA | Yes | No | Suggests use only if CEA was elevated pre-treatment |
| ESMO (Europe) | CEA, CA 19-9 | Yes (in select cases) | No | Encourages marker + imaging combination |
| Japanese Guidelines | CEA, CA 19-9 | Yes | No | Includes CEA in post-op risk models |
As seen, no guideline recommends tumor markers alone for diagnosis or population-wide screening. Their strength lies in recurrence monitoring and therapy guidance—especially when interpreted in the context of symptoms and imaging.
Limitations and False Positives of Tumor Markers
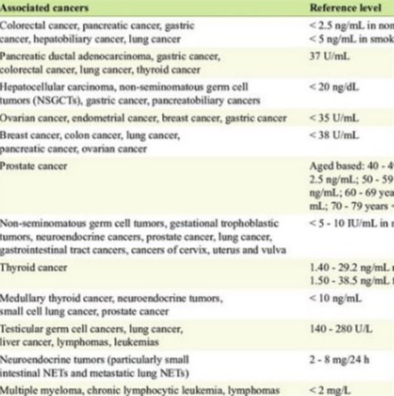
While tumor markers are powerful tools, they are not without flaws. One major issue is false positives—elevated levels that do not indicate cancer. For example, CEA may be elevated in smokers, patients with liver disease, or inflammatory bowel conditions.
Other sources of false positives include:
- CA 19-9: Can be high in pancreatitis or bile duct obstruction
- Inflammatory states: General inflammation may mildly raise some markers
- Laboratory variability: Results can vary slightly across labs and methods
Relying solely on markers without imaging or clinical context may lead to unnecessary anxiety or procedures. That’s why oncologists interpret markers in trend, not isolation.
Educating patients about this helps reduce panic when one value is elevated. It’s the overall clinical picture that determines action.
Tumor Markers and Personalized Medicine
In recent years, tumor marker testing has moved beyond traditional proteins like CEA. Now, genetic and molecular markers are part of precision oncology. These include:
- KRAS, NRAS, and BRAF mutations
- Microsatellite instability (MSI) status
- Circulating tumor DNA (ctDNA)
These tests not only help predict recurrence, but also guide targeted therapy. For instance, BRAF-mutated bowel cancer may respond to specific inhibitors, while MSI-high cancers may benefit from immunotherapy.
Some clinical trials now use ctDNA to detect relapse months before imaging, creating a new wave of early intervention strategies.
These innovations bridge the gap between tumor markers and personalized treatment, making follow-up more accurate and therapy more tailored.
Comparing Bowel Cancer Markers with Other Conditions
To understand the specificity of tumor markers in bowel cancer, it helps to compare them with other conditions. Here’s a breakdown:
| Condition | Common Marker | Specific to Bowel Cancer? | Notes |
| Bowel Cancer | CEA | Yes, but not exclusively | Best in recurrence monitoring |
| Pancreatic/Bile Duct Cancer | CA 19-9 | No | Elevated in other GI conditions too |
| Liver Disease | CEA, CA 19-9 | No | Elevation may mislead |
| Inflammatory Bowel Disease | CEA (mild) | No | Inflammation can raise levels slightly |
| Lung Cancer (advanced) | CEA | Sometimes | Non-specific marker, must be used with caution |
Patients worried about overlapping symptoms—like whether bowel leakage is a sign of cancer—should rely on the full diagnostic pathway, not just marker testing. Markers are a part of the equation, not the answer on their own.
Patient Tips: Understanding and Using Tumor Marker Tests
Here are insights for patients navigating tumor marker discussions with their care team:
- Always ask for your baseline: Know your starting level after treatment
- Track trends: One result means little; what matters is change over time
- Communicate symptoms: Especially if subtle issues like fatigue or symptoms of bowel cancer recur
- Discuss timing: Ask how often tests will be done and why
- Understand limits: No marker is perfect. Normal doesn’t mean guaranteed safety, and elevated doesn’t always mean cancer
Empowered patients who understand the role of tumor markers can participate in shared decision-making and reduce anxiety around testing.
Limitations of Tumor Markers in Bowel Cancer Diagnosis
While tumor markers are useful tools, they come with notable limitations that must be understood, especially by patients relying on them for early diagnosis. One of the major issues is that tumor markers like CEA (carcinoembryonic antigen) and CA 19-9 are not exclusive to bowel cancer. Elevated levels may also appear in benign conditions such as inflammatory bowel disease, pancreatitis, or even after smoking. This makes them unreliable as stand-alone screening tools.
Moreover, not all bowel cancer patients exhibit elevated tumor marker levels. In early stages of the disease, markers may remain within the normal range, giving false reassurance. Some tumors may simply not secrete these antigens in measurable quantities. Hence, these tests must be interpreted alongside colonoscopy results, histopathology, and radiographic imaging for a more complete picture.
How Tumor Markers Are Used in Treatment Monitoring
Tumor markers become especially valuable during and after treatment for bowel cancer. Once a patient has a confirmed diagnosis, baseline levels are measured. After surgery or chemotherapy, subsequent tests can detect a significant drop in marker concentration, which often correlates with successful response to treatment.
Doctors also use tumor marker trends to identify potential recurrence. A rising CEA level post-treatment can signal that cancer may be returning—sometimes even before symptoms or imaging changes appear. This can trigger early investigations or even preemptive intervention. Still, it’s critical to avoid overreaction to a single elevated result; trends over time offer more meaningful insights than isolated readings.
Emerging Tumor Markers and Molecular Biomarkers
Traditional tumor markers are gradually being supplemented—or even replaced—by newer molecular biomarkers. These include DNA methylation markers, circulating tumor DNA (ctDNA), and specific gene mutations such as KRAS, BRAF, and MSI (microsatellite instability). These emerging tools provide more targeted, accurate insights into both diagnosis and prognosis.
For instance, detecting microsatellite instability not only suggests a subtype of colorectal cancer but also indicates likely resistance to certain chemotherapy agents, while pointing toward immunotherapy as a better alternative. Liquid biopsies using ctDNA are gaining traction because they allow detection of genetic alterations non-invasively. These advancements enhance personalization of bowel cancer treatment plans and improve long-term outcomes.
Integrating Tumor Marker Testing into Personalized Care
The real power of tumor markers lies in their integration into a broader strategy of personalized medicine. Oncologists now combine these markers with histologic subtype, genetic mutations, and imaging data to tailor treatment. For example, a patient with high CEA and a detected KRAS mutation might be excluded from certain biologic therapies and steered toward more promising options.
This integrative approach also helps refine surveillance plans. Someone with low-risk pathology and normal tumor markers may not require intensive imaging every few months, whereas a patient with persistently elevated markers might need closer observation.
Understanding how tumor markers fit into this framework can ease anxiety and empower patients to make informed decisions in collaboration with their medical team. For more insight into early warning signs, see our resource on Symptom of Bowel Cancer.
FAQ: Bowel Cancer Tumor Markers
1. Can tumor markers detect bowel cancer before symptoms appear?
From a clinical standpoint, tumor markers like CEA are generally not reliable for detecting bowel cancer in its early, asymptomatic stages. While they can occasionally show elevations, especially in advanced disease, many early-stage tumors do not produce enough marker substances to trigger alerts. Therefore, they are not recommended as primary screening tools. Colonoscopy remains the gold standard.
2. Is it possible to have normal tumor markers but still have cancer?
Yes, absolutely. Many patients with bowel cancer — particularly in its early stages — may present with completely normal levels of CEA or CA 19-9. That’s why relying solely on blood tests for diagnosis is risky. Tumor markers must always be interpreted alongside imaging and biopsy data.
3. How often are tumor markers measured during treatment?
Typically, oncologists check tumor markers before treatment begins to establish a baseline. Then they may repeat the tests every 1–3 months during chemotherapy or after surgery to evaluate the response. After treatment completion, monitoring continues every 3–6 months depending on the recurrence risk profile.
4. What’s considered a “high” CEA level?
Normal CEA levels in non-smokers are usually less than 3 ng/mL, while smokers may have levels up to 5 ng/mL. Anything significantly higher may raise suspicion — particularly if levels continue to rise over time — but interpretation depends on the context and trends, not just one number.
5. Can tumor markers be used to determine the stage of bowel cancer?
No, tumor markers alone cannot determine the stage. While elevated levels may suggest more advanced disease, true staging requires a combination of imaging (like CT scans), endoscopy, and histopathologic analysis of tumor tissue.
6. Are tumor marker levels affected by other conditions?
Yes, several benign conditions such as liver disease, ulcers, pancreatitis, and even infections can raise levels of markers like CEA and CA 19-9. That’s why elevated levels do not automatically mean cancer — they’re only part of the overall diagnostic picture.
7. What are circulating tumor DNA (ctDNA) tests, and are they better?
ctDNA tests analyze small fragments of tumor DNA in the blood. They offer a more precise, real-time view of a tumor’s genetic profile and may detect recurrence before conventional markers do. While still under evaluation in clinical settings, ctDNA is a promising addition to bowel cancer monitoring.
8. Can lifestyle changes affect tumor marker levels?
Not directly. However, quitting smoking can normalize slightly elevated CEA levels in otherwise healthy individuals. Diet or exercise typically don’t influence marker levels once cancer is present.
9. What happens if tumor markers stay high after surgery?
Persistent elevation of tumor markers post-surgery may indicate residual disease or early recurrence. Your care team will usually follow up with imaging (CT or PET scans) and possibly additional biopsies to investigate further.
10. Are tumor markers useful in screening for hereditary bowel cancer?
No. Individuals with familial cancer syndromes like Lynch syndrome should undergo genetic testing and routine colonoscopy. Tumor markers are not useful as screening tools for hereditary risk alone.
11. Do tumor markers differ in rectal vs. colon cancer?
The markers themselves — such as CEA — are the same for both rectal and colon cancers. However, response patterns may vary slightly, and the interpretation of marker trends should be individualized.
12. Can stress or infection cause tumor markers to spike?
Minor fluctuations may occur, but large spikes are unlikely to result from stress alone. Infections or inflammatory conditions can temporarily raise markers like CA 19-9, so repeat testing is often done after resolution of these issues.
13. What’s the connection between bowel marker levels and treatment choices?
High tumor marker levels may signal more aggressive disease, prompting consideration of chemotherapy even after surgery. They may also indicate whether biologic therapies are appropriate depending on the presence of gene mutations.
14. Are tumor markers ever used in IBS patients?
Not typically. IBS (irritable bowel syndrome) is a functional disorder without structural abnormalities or tumor development. Still, because symptoms can overlap with early cancer, diagnostic testing may include a CEA test if red flags arise.
15. Can tumor markers predict survival in bowel cancer?
Elevated tumor markers at diagnosis — especially very high levels — are often associated with poorer prognosis. However, many other variables impact survival, including stage, response to treatment, and overall health. These levels are part of the risk stratification process, not a definitive prediction.