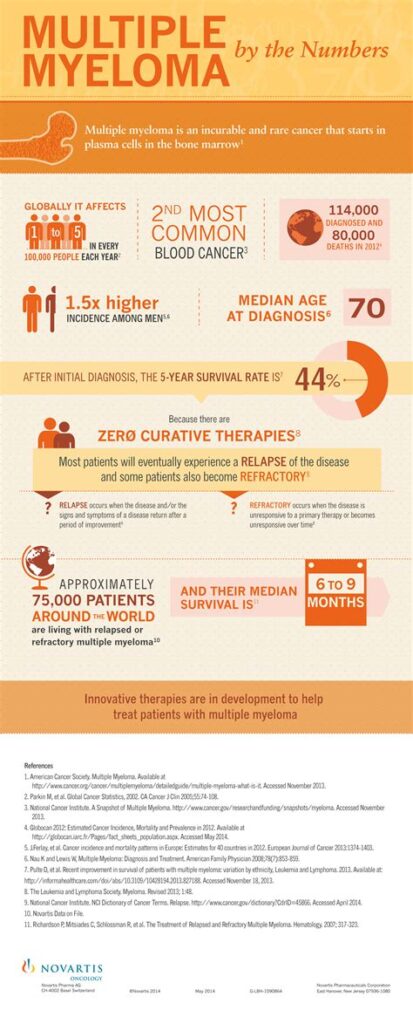
Multiple Myeloma as a Hereditary Cancer
Multiple Myeloma as a Hereditary Cancer: What You Need to Know
- What Is Multiple Myeloma and How Does It Affect the Body
- Understanding Hereditary Cancer and Genetic Susceptibility
- What Research Tells Us About Genetic Links to Myeloma
- Symptoms That Could Point to Hereditary Multiple Myeloma
- How Multiple Myeloma Is Diagnosed in High-Risk Families
- When Should Genetic Counseling Be Considered?
- Can Preventive Measures Lower the Risk of Hereditary Myeloma?
- Treatment Approaches for Hereditary Cases of Multiple Myeloma
- Living with a Hereditary Risk of Multiple Myeloma
- Should Other Family Members Be Screened?
- BRCA Mutations and Their Role in Blood Cancers
- Coping with Emotional and Mental Health Burdens
- How Future Treatments May Target Genetic Pathways
- Epigenetics: Why Your Genes Aren’t Destiny
- Comparing Hereditary and Sporadic Multiple Myeloma
- How Genetic Testing Is Reshaping Cancer Prognosis
- 15+ Frequently Asked Questions
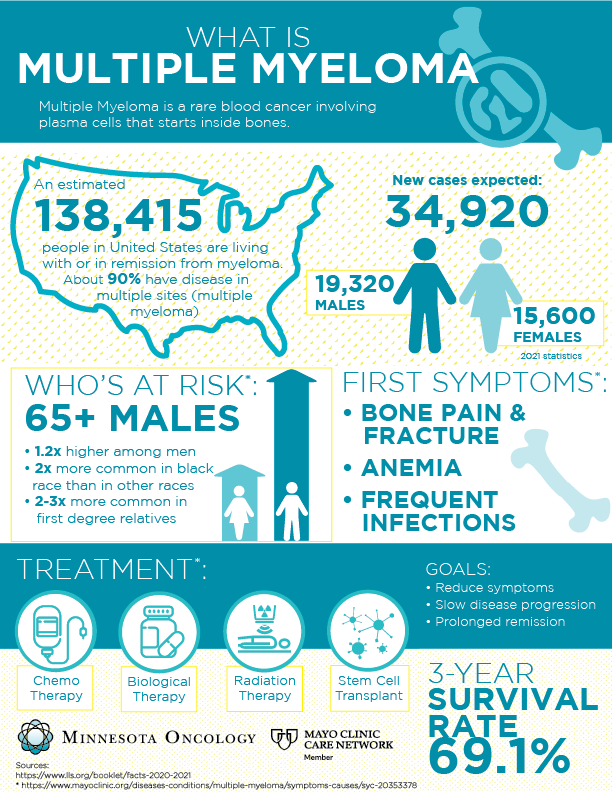
What Is Multiple Myeloma and How Does It Affect the Body
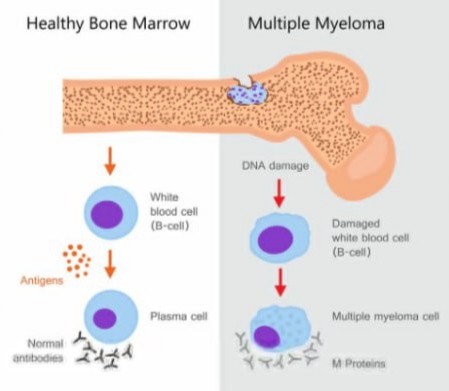
Multiple myeloma is a type of blood cancer that begins in plasma cells, a form of white blood cells made in the bone marrow. Plasma cells are crucial for producing antibodies that help fight infections. In multiple myeloma, these cells mutate, multiply uncontrollably, and crowd out healthy cells. This leads to weakened immunity, fragile bones, kidney problems, and anemia.
Unlike cancers that form solid tumors, myeloma spreads throughout the bone marrow in different bones simultaneously. This makes it a “systemic” cancer, often leading to generalized bone pain, particularly in the back, hips, and ribs. The disease is typically diagnosed in people over 60, but hereditary forms can emerge earlier.
The condition is considered incurable but manageable with the right therapy. Understanding whether it runs in families helps determine screening needs and early intervention options.
Understanding Hereditary Cancer and Genetic Susceptibility
Hereditary cancers arise when genetic mutations passed down from parents increase the risk of specific malignancies. In multiple myeloma, some individuals may inherit gene variants that elevate their lifetime cancer risk compared to the general population.
This doesn’t mean a person will definitely develop cancer, but it does increase susceptibility. In the case of multiple myeloma, research suggests that close relatives of patients—particularly siblings or children—have a slightly higher incidence of the disease.
Below is a breakdown showing the general difference between hereditary vs. non-hereditary cancer origins:
| Factor | Hereditary Myeloma | Sporadic Myeloma |
| Family History | One or more affected relatives | Typically none |
| Age of Onset | Often younger (<60 years) | Usually over 65 |
| Gene Mutations Involved | Germline mutations (inherited) | Somatic mutations (acquired) |
| Recurrence Risk in Family | Elevated | Low |
| Preventive Screening Recommended | Yes | Case-dependent |
The most studied genes in this regard include BRCA1, BRCA2, and rare familial syndromes affecting immune regulation and bone marrow health.
What Research Tells Us About Genetic Links to Myeloma
Although multiple myeloma is not classically labeled a “hereditary cancer,” modern research continues to explore how family history and genetic variants play a role. Genome-wide association studies (GWAS) have identified specific inherited polymorphisms that may increase the likelihood of developing the disease.
For example, variants in regions such as 3p22.1, 7p15.3, and 17p11.2 have been implicated. These gene regions are involved in immune regulation, cell division, and DNA repair—core functions that, when disrupted, may lead to cancer development.
A family member with multiple myeloma does not guarantee inheritance, but it warrants closer attention. If you have more than one relative affected, or if cases occurred before age 50, it might be time to consult a genetic counselor.
This ties closely to broader discussions on hereditary blood cancers like those found in prostate cancer cases.
Symptoms That Could Point to Hereditary Multiple Myeloma
Many symptoms of multiple myeloma overlap with non-hereditary cases, but a key factor is early onset or severity at diagnosis. Watch for:
- Persistent bone pain, especially in the back or ribs
- Frequent infections
- Excessive fatigue not relieved by rest
- Bruising or bleeding easily
- Unexplained weight loss
- Tingling or numbness in the legs (due to nerve compression)
If these signs develop in someone under age 50, and there’s a family history of blood disorders or cancers, this may suggest a hereditary component. More details on early diagnostic measures will be provided in the next section.
How Multiple Myeloma Is Diagnosed in High-Risk Families
When multiple myeloma is suspected—especially in someone with a strong family history—doctors pursue a more targeted diagnostic approach. This may begin with a combination of blood tests, imaging, bone marrow biopsy, and genetic screening.
Key diagnostic tools include:
| Test Name | Purpose |
| Serum Protein Electrophoresis (SPEP) | Detects abnormal M protein in the blood |
| Immunofixation Electrophoresis | Confirms and classifies the M protein |
| Free Light Chain Assay | Measures light chain ratios in the blood |
| Bone Marrow Biopsy | Confirms plasma cell cancer |
| MRI or PET-CT | Identifies bone lesions or organ involvement |
| Genetic Testing (e.g., BRCA, TP53) | Assesses inherited risk factors |
In individuals from families with a known history, genetic testing might occur even before symptoms appear. Early detection is crucial not only for extending survival but for improving quality of life and reducing irreversible complications like kidney failure or spinal fractures.
When Should Genetic Counseling Be Considered?
Genetic counseling is an essential tool for families with suspected hereditary cancer syndromes. A certified genetic counselor helps patients and relatives understand whether their family tree suggests an inherited risk of myeloma or related cancers.
Who should consider genetic counseling:
- People with two or more first-degree relatives with multiple myeloma or related blood cancers
- Individuals diagnosed under age 50
- Families with mixed cancers across generations (e.g., breast, prostate, blood cancers)
- Patients with known mutations (e.g., BRCA2) related to myeloma development
Genetic counselors may recommend targeted gene panel testing. This has grown more affordable and accessible over the past decade. Counseling also helps interpret results that are not straightforward—such as a “variant of uncertain significance” (VUS), which doesn’t confirm nor rule out risk.
Family members who test positive may also be eligible for more frequent blood screenings and imaging, potentially allowing for pre-cancer detection or early treatment planning.
Can Preventive Measures Lower the Risk of Hereditary Myeloma?
There’s no way to fully “prevent” multiple myeloma, even in high-risk individuals. But several steps can reduce the chance of triggering cancer pathways, especially in those with a genetic predisposition.
These include:
| Protective Action | Impact on Risk |
| Avoiding exposure to benzene and pesticides | Lowers environmental contribution |
| Maintaining a healthy BMI | Reduces chronic inflammation and stress signals |
| Quitting smoking | Lowers DNA damage from carcinogens |
| Regular check-ups with blood tests | Allows early identification of clonal changes |
| Vitamin D optimization | Supports immune surveillance |
Ongoing research is also evaluating chemoprevention agents—drugs taken by high-risk individuals to reduce the chance of cancer starting. This is similar to how tamoxifen is used in high-risk breast cancer populations.
It’s worth noting that improving early detection among genetically predisposed patients may have even greater impact than prevention.
Treatment Approaches for Hereditary Cases of Multiple Myeloma
Treatment for hereditary multiple myeloma is largely the same as for sporadic cases. However, the treatment timeline often begins earlier, and long-term planning becomes more important for younger patients who may live with the disease for decades.
Typical frontline therapies include:
- Immunomodulatory drugs (IMiDs): lenalidomide, thalidomide
- Proteasome inhibitors: bortezomib, carfilzomib
- Monoclonal antibodies: daratumumab, elotuzumab
- Autologous stem cell transplantation (ASCT)
- Corticosteroids: dexamethasone
Here is a comparative overview:
| Therapy Type | Goal | Suitability for Hereditary Cases |
| Induction Therapy | Rapidly reduce cancer burden | Standard, regardless of heredity |
| Stem Cell Transplant | Deep remission | More often used in younger patients |
| Maintenance Therapy | Prolong remission | Essential in long-term hereditary cases |
| Clinical Trials | Test novel, targeted agents | Encouraged for high-risk genotypes |
Many hereditary patients may benefit from personalized treatments that factor in their genetic profile. For example, individuals with BRCA-related mutations may respond better to PARP inhibitors, a class of drugs already used in breast and ovarian cancers.
This also connects with ongoing studies on Sermorelin side effects and cancer development, particularly regarding hormone-modulated growth pathways.
Living with a Hereditary Risk of Multiple Myeloma
Living with a genetic predisposition to multiple myeloma means adopting both a proactive health strategy and a long-term mindset. People with hereditary risk often live without symptoms for years—but that doesn’t mean they shouldn’t prepare.
The key is risk-informed wellness, which includes:
- Building a consistent relationship with a hematologist or oncologist
- Scheduling periodic bloodwork even in the absence of symptoms
- Keeping a personal health journal that logs fatigue, infections, and bone pain
- Communicating known genetic risks with all healthcare providers
Psychologically, the challenge is balancing awareness with optimism. Many people struggle with “what-if” anxiety or feel isolated in families affected by cancer. Peer support groups or genetic counseling services can help normalize the experience and build emotional resilience.
This kind of preparedness becomes especially important when early signs—such as frequent infections or unexplained back pain—begin to emerge.
Should Other Family Members Be Screened?
Yes, family screening is strongly encouraged in cases where multiple myeloma or similar blood cancers appear across generations. This can uncover other at-risk relatives who may benefit from early testing, lifestyle guidance, or preventive care.
Screening often includes:
| Family Member | Recommended Screening |
| Siblings of the patient | Full genetic panel + regular blood tests after age 40 |
| Children of the patient | Genetic counseling by age 25–30 (sooner if symptomatic) |
| Parents | Genetic counseling and age-adjusted testing |
This approach mirrors what is already done for breast or colorectal cancer families, where early testing has saved lives.
In some cases, testing may reveal related hereditary syndromes, such as Li-Fraumeni or BRCA-associated malignancies. This would also require referral to specialists for management, possibly linking to Is Myeloma Cancer Hereditary.
BRCA Mutations and Their Role in Blood Cancers
Although BRCA1 and BRCA2 mutations are typically associated with breast and ovarian cancers, research has shown that they may also contribute to the development of multiple myeloma. The mechanisms are tied to their function in DNA repair—when this pathway fails, abnormal plasma cells can accumulate unchecked.
Recent studies reveal:
- BRCA2 mutations are more commonly linked to myeloma than BRCA1
- BRCA-positive patients may respond better to certain targeted therapies
- These mutations increase the likelihood of multiple cancers appearing in a single family
Here is a comparison:
| Mutation | Commonly Associated Cancers | Relevance to Myeloma |
| BRCA1 | Breast, ovarian, pancreatic | Emerging evidence; less frequent |
| BRCA2 | Breast, ovarian, prostate, blood cancers | Stronger evidence in multiple myeloma |
This overlap also highlights the need to view genetic mutations through a broad cancer lens. A patient referred for Intermediate-Risk Prostate Cancer due to BRCA2 status may also benefit from blood cancer surveillance.
Coping with Emotional and Mental Health Burdens
The emotional side of hereditary cancer risk can’t be underestimated. Patients who know they carry cancer-prone genes often feel like they’re “waiting for it to happen.” This uncertainty can fuel anxiety, depression, and relationship tension.
Key mental health strategies include:
- Meeting with oncology-specific therapists who understand the hereditary burden
- Mindfulness techniques to manage anxiety around screening results
- Participating in genetic counseling sessions with the whole family
- Creating a structured “what-if” action plan to reduce helplessness
Building a care team that includes mental health professionals is just as important as oncologists and geneticists. Hereditary risk isn’t just physical—it deeply affects identity and future planning.
How Future Treatments May Target Genetic Pathways
As genetic understanding deepens, so does the potential for targeted therapies that work precisely with a patient’s inherited mutations. In multiple myeloma, this means leveraging knowledge of DNA repair, immune response, and cellular signaling to create personalized medicine.
New approaches under clinical investigation include:
- PARP inhibitors for BRCA-related myeloma, which block tumor repair mechanisms
- CAR T-cell therapy tuned to familial myeloma profiles
- Checkpoint inhibitors that exploit immune vulnerabilities specific to inherited cancers
Here’s a simplified table showing how mutation type may guide treatment direction:
| Mutation Type | Targeted Therapy Example | Development Status |
| BRCA2 | PARP inhibitors (e.g., olaparib) | In trials for myeloma |
| TP53 or ATM | Immune checkpoint inhibitors | Under study |
| RUNX1 mutations | Gene-editing/CRISPR approaches | Early experimental phase |
The integration of Sermorelin Side Effects and Cancer has also entered discussions, as hormonal pathways can affect how fast genetically driven cancers progress—especially in patients with endocrine sensitivity.
Epigenetics: Why Your Genes Aren’t Destiny
It’s now clear that epigenetics—how genes are turned on or off—plays a critical role in myeloma development. This explains why some individuals with known mutations never develop cancer, while others do despite negative family histories.
Factors influencing gene expression include:
- Chronic inflammation
- Environmental toxins
- Stress and trauma
- Microbiome imbalance
Epigenetic changes don’t mutate the DNA code itself but alter how cells interpret and execute it. For instance, silencing a tumor suppressor gene can promote myeloma even if no formal mutation exists.
This opens the door to preventive therapies using lifestyle, diet, supplements, or epigenetic drugs, aiming to keep harmful genes inactive.
Comparing Hereditary and Sporadic Multiple Myeloma
While the disease may appear similar in both hereditary and non-hereditary (sporadic) cases, there are important distinctions:
| Factor | Hereditary Myeloma | Sporadic Myeloma |
| Age of onset | Earlier (40s–50s) | Later (60s–70s) |
| Family history | Often present | Rarely present |
| Mutation involvement | BRCA, TP53, ATM common | Not usually genetically linked |
| Prognosis variability | Depends on mutation type | Tied more to stage/response |
| Screening relevance | Strongly recommended for family | Not usually advised for family |
Understanding these differences helps in tailoring treatment, monitoring, and genetic counseling strategies. It also encourages high-risk individuals to get ahead of the disease via regular testing and risk-reduction strategies.
How Genetic Testing Is Reshaping Cancer Prognosis
Genetic testing no longer serves just as a diagnostic tool—it has become a predictive and therapeutic cornerstone. For multiple myeloma, this means earlier detection, better risk stratification, and smarter treatment choices.
There are three types of tests relevant here:
| Test Type | Purpose |
| Germline testing | Identifies inherited mutations |
| Somatic testing | Examines mutations within tumor cells |
| Panel testing | Screens for a group of cancer-related mutations |
When these tests reveal mutations, they offer insight into:
- Recurrence likelihood
- Suitability for bone marrow transplant
- Potential for developing secondary cancers
For patients, this means more personalized care. For doctors, it improves decision-making. For families, it informs whether relatives should test and take preventive steps.
15+ Frequently Asked Questions
1. Can multiple myeloma skip generations?
Yes, it can. Hereditary cancer risk is passed genetically, but not everyone who inherits a mutation will develop the disease. This is due to gene penetrance and environmental or epigenetic factors that influence expression.
2. Is there a blood test that can detect inherited myeloma risk?
There is no single blood test that confirms risk. However, germline genetic testing from blood or saliva samples can detect inherited mutations associated with multiple myeloma.
3. Does a BRCA mutation mean I will definitely get multiple myeloma?
No. A BRCA mutation increases your risk, but many people with BRCA1 or BRCA2 mutations never develop myeloma or any cancer. Lifestyle, environment, and other genes also play a role.
4. Is hereditary multiple myeloma more aggressive?
It depends on the specific mutation. For example, TP53-related myeloma tends to be more aggressive. But in general, disease behavior varies significantly based on genetics and treatment response.
5. Can I reduce my risk if I have a family history?
Yes. While you can’t change your genes, you can influence how they are expressed. Diet, avoiding toxins, monitoring for early signs, and reducing inflammation are helpful preventive measures.
6. How early can multiple myeloma be detected in genetic carriers?
Routine screening in high-risk individuals can detect precursor conditions like MGUS or smoldering myeloma before full-blown disease develops. Some cases are identified as early as age 30–40.
7. Are men or women more likely to inherit myeloma risk?
Both sexes can inherit the same mutations. However, men are statistically more likely to develop multiple myeloma, regardless of hereditary status.
8. Should I tell family members if I test positive for a mutation?
Yes. Sharing genetic results helps relatives assess their own cancer risk and make decisions about testing, surveillance, or preventive measures.
9. Can lifestyle reverse inherited myeloma risk?
While it can’t reverse your genetic makeup, lifestyle choices can dramatically reduce the likelihood of gene expression turning into active disease, especially in epigenetically influenced mutations.
10. What cancers are related to the same genes as myeloma?
Genes like BRCA2, ATM, and TP53 are also linked to breast, prostate, pancreatic, and ovarian cancers. So a family history of these cancers may also indicate myeloma risk.
11. What’s the role of bone marrow biopsies in hereditary cases?
Bone marrow biopsies are used to confirm diagnosis or monitor progression, but genetic predisposition is usually detected through DNA analysis, not biopsies.
12. How often should someone with a mutation get checked?
High-risk individuals are often monitored annually or biannually, depending on family history, test results, and the presence of precancerous markers like MGUS.
13. Can children be tested for myeloma mutations?
Technically yes, but most experts recommend waiting until adulthood unless there is a compelling clinical reason. This allows informed consent and psychological readiness.
14. Are there clinical trials for hereditary myeloma?
Yes. Trials increasingly target patients with specific mutations. Ask about genetically matched therapies or preventive trials at academic centers or through national cancer databases.
15. Is there a difference between inherited and acquired mutations in myeloma?
Yes. Inherited (germline) mutations are present in all cells and passed from parents. Acquired (somatic) mutations occur spontaneously in cells during life and are not passed on.