Updated List of Cancer Symptoms in 2025
By 2025, oncologists and general practitioners have significantly refined their understanding of how cancer manifests—not just in advanced cases, but increasingly in early-stage and subclinical presentations. Where once we relied on physical signs such as a palpable lump or visible bleeding, we now recognize the diagnostic value of more nuanced symptoms: a slight drop in hemoglobin, persistent fatigue, or subtle inflammatory activity in lab work.
This updated guide aims to provide a detailed look at what we currently recognize as red flags for malignancy, integrating both subjective symptoms and objective medical data, with emphasis on timing, persistence, and context. Cancer rarely announces itself loudly at first. It whispers. And those who learn to listen early are often the ones who save lives.
General Systemic Symptoms of Cancer
The most insidious aspect of systemic cancer symptoms is that they are often mistaken for “just life.” Fatigue after work. Weight loss due to stress. A low-grade fever dismissed as seasonal. However, when such nonspecific complaints persist or appear in clusters, they may represent the early metabolic footprint of malignancy.
Clinically, persistent fatigue that is not relieved by rest remains one of the most reported complaints in early cancer diagnoses. It often precedes other findings by weeks or even months. In patients with no obvious explanation—no depression, no thyroid disorder, no iron deficiency—this symptom alone warrants attention, particularly in combination with low-grade fever or appetite change.
Weight loss exceeding 5% of baseline body mass over several months, especially when unintentional and not accompanied by increased physical activity, is another recognized hallmark. When paired with subtle laboratory signals—such as a mild drop in hemoglobin or a rise in C-reactive protein (CRP)—the concern deepens. These “silent markers” reflect the systemic inflammation or tumor-induced metabolic changes that often occur long before imaging reveals the cause.
In clinical experience, one of the most overlooked combinations is night sweats + borderline anemia + high ESR, especially in younger adults. While not specific, this triad is frequently encountered in low-grade lymphomas and often missed until much later.
Laboratory markers that support systemic suspicion include:
- Mild normocytic anemia, particularly when iron levels are not clearly deficient
- Elevated LDH (lactate dehydrogenase), especially in aggressive or hematologic cancers
- Thrombocytosis or unexplained drop in platelet count
- Slight hyponatremia or hypercalcemia, sometimes driven by paraneoplastic syndromes
When these markers coincide with non-resolving fatigue, subtle weight loss, or nonspecific discomfort (such as bone aches, low appetite, or “not feeling right”), the clinician should begin ruling out occult malignancy, even in the absence of focal findings.
Organ-Specific Clues and the Challenge of Attribution
In early cancer detection, location-specific symptoms are notoriously misleading—precisely because they mimic benign conditions. The challenge lies in identifying when common complaints behave uncommonly.
Take, for example, persistent cough. In smokers over 50, it’s expected. But when that cough doesn’t respond to treatment, becomes more hoarse, or is accompanied by trace blood in phlegm—even with normal chest x-rays—it could reflect central airway involvement from an early bronchogenic carcinoma.
Similarly, a change in bowel habits, such as alternating constipation and diarrhea or a feeling of incomplete evacuation, is frequently attributed to diet or stress. But in patients over 40, particularly those with iron-deficiency anemia or a family history of colorectal cancer, these “functional” symptoms demand further workup—often via colonoscopy—even in the absence of rectal bleeding.
In breast cancer, women are frequently taught to look for a lump, yet many first notice skin texture changes or a sensation of heaviness. Inflammatory subtypes may present with redness and swelling before a mass is even detectable.
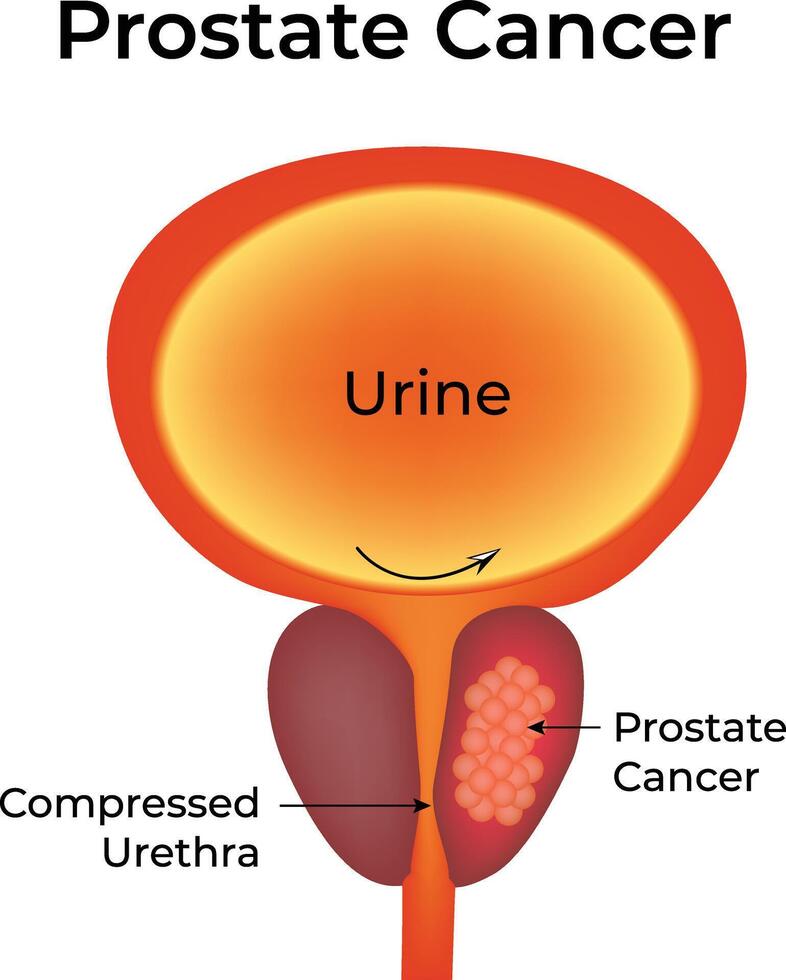
In male patients with prostate cancer, symptoms such as urinary hesitancy or nocturia are often dismissed as normal aging. However, subtle signs like reduced urine stream force or post-void dribbling—especially in combination with a PSA rise—may be early indicators of underlying malignancy.
For gastric and pancreatic cancers, early signs tend to be digestive and vague: fullness after small meals, minor nausea, mild discomfort under the ribs. But when paired with recent weight loss, this constellation gains meaning.
One critical point in 2025 clinical thinking: any persistent, unexplained symptom lasting more than 4–6 weeks in an adult over 40 should trigger diagnostic curiosity, particularly if accompanied by laboratory or constitutional warning signs.
Blood Test Abnormalities That May Indicate Cancer
While no blood test can diagnose cancer with certainty, specific hematologic and biochemical abnormalities often serve as early indicators that something may be wrong. In many cases, these changes precede imaging findings or clinical symptoms. As of 2025, oncologists rely on subtle deviations across multiple parameters rather than a single alarming value.
Anemia remains one of the most common findings in undiagnosed cancer patients. It may be normocytic (normal cell size), microcytic (typically iron-deficiency), or macrocytic (vitamin B12-related, sometimes paraneoplastic). In gastrointestinal cancers—especially colon and gastric—the anemia is often iron-deficiency in origin, secondary to occult bleeding. In contrast, in lymphomas and bone marrow malignancies, the anemia is typically normocytic and associated with inflammatory markers.
Another red flag is elevated platelet count (thrombocytosis), which is often reactive in nature and associated with tumors of the lung, gastrointestinal tract, and female reproductive system. While frequently overlooked, sustained thrombocytosis in the absence of infection or inflammation should prompt oncologic screening.
Elevations in LDH (lactate dehydrogenase) often signal high cell turnover and tissue breakdown. It is especially relevant in lymphomas, melanomas, and testicular cancers, and is also used for staging and prognosis.
Here’s a simplified but clinically relevant reference table:
| Marker | Possible Malignancy Associations | Commentary |
|---|---|---|
| Hemoglobin ↓ | Colon, gastric, kidney, bone marrow cancers | Rule out GI bleeding, marrow infiltration |
| Platelets ↑ | Lung, ovarian, colon, endometrial cancers | May precede diagnosis by months |
| LDH ↑ | Lymphoma, melanoma, testicular, small cell lung | Prognostic marker in lymphoma staging systems |
| CRP, ESR ↑ | Almost all advanced malignancies | Reflects tumor-related inflammation |
| Ferritin ↑ | Liver cancer, leukemia, inflammatory-driven tumors | Must be interpreted alongside iron and transferrin levels |
| WBC ↑ or ↓ | Leukemias, lymphomas | Extreme leukocytosis or cytopenias always warrant workup |
| Calcium ↑ | Lung, breast, myeloma (via PTHrP secretion) | Paraneoplastic hypercalcemia can be life-threatening |
| D-dimer ↑ | Pancreatic, lung, brain, advanced-stage cancers | Often a marker of occult coagulopathy or metastasis |
What’s vital in modern oncology is context. A single high CRP doesn’t mean cancer. But high CRP + anemia + elevated LDH in a 60-year-old with unintentional weight loss? That’s a red flag.
In 2025, Artificial Intelligence tools integrated into lab systems are being used in some hospitals to flag such combinations and suggest further diagnostic pathways, including imaging or referral to oncology.
Neurological and Cognitive Symptoms Associated with Cancer
Cancer is not just a physical illness of masses and markers—it is also a neurological disruptor, capable of mimicking psychiatric disorders, triggering seizures, and causing debilitating syndromes even when no tumor is present in the brain.
In some patients, subtle cognitive changes—such as difficulty concentrating, word-finding pauses, or short-term memory loss—may be the only early symptoms. While often misattributed to stress or aging, they can reflect paraneoplastic neurologic syndromes (PNS), where the immune system, triggered by cancer, attacks parts of the nervous system.
Other patients may present with peripheral neuropathy, typically described as burning or tingling in the hands and feet. This can be a paraneoplastic sign or a direct result of myeloma or neuroendocrine tumors. In elderly patients, a newly diagnosed “idiopathic” neuropathy should always prompt protein electrophoresis to rule out myeloma or monoclonal gammopathy.
More dramatically, patients may develop seizures, gait instability, or focal neurological deficits. In such cases, brain metastases or primary CNS lymphomas must be excluded via MRI.
One of the most overlooked syndromes in clinical practice is Lambert-Eaton Myasthenic Syndrome (LEMS). Patients present with progressive muscle weakness, especially in the legs. It is strongly associated with small cell lung cancer and often appears before the tumor is even visible on imaging.
Another condition, anti-NMDA receptor encephalitis, can occur in young females with ovarian teratomas. The presentation mimics schizophrenia or bipolar disorder—hallucinations, mania, catatonia—leading to frequent misdiagnosis in psychiatric settings.
What makes these syndromes terrifying—and crucial to recognize—is that the cancer causing them is often small and otherwise asymptomatic. In some cases, curing the underlying tumor cures the syndrome. In others, the neurological damage is irreversible unless caught early.
By 2025, neurologists and oncologists increasingly collaborate on “red flag neurology,” where subtle CNS signs are interpreted as potential harbingers of cancer, particularly when standard workups (B12, MRI, thyroid) are normal.
Cancer Symptoms by Tumor Type
In 2025, the classification of cancer-related symptoms increasingly relies not only on anatomical site, but on tumor biology, immune interaction, and molecular behavior. However, many classic symptom patterns remain, and clinicians continue to group signs by tumor origin for initial diagnostics.
Gastrointestinal Cancers
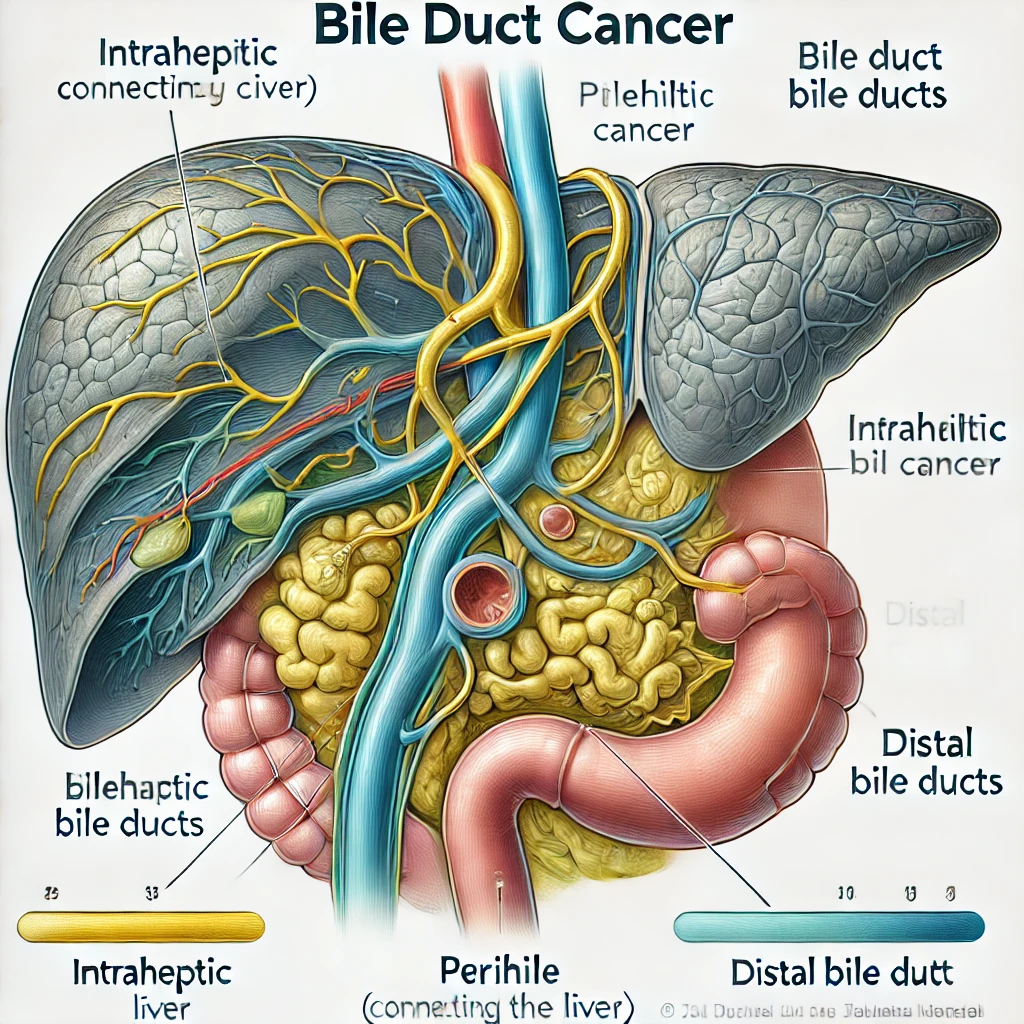
GI cancers, including esophageal, gastric, colon, liver, and pancreatic, often develop silently. Early symptoms include vague abdominal discomfort, changes in bowel habits (alternating constipation and diarrhea), and unintended weight loss. Occult GI bleeding—manifesting as iron-deficiency anemia—is a red flag, especially in men over 50 or postmenopausal women. Pancreatic cancer may present with painless jaundice, back pain, or newly diagnosed diabetes in older adults.
Lung Cancer
Lung cancer is notorious for masquerading as benign respiratory conditions. A persistent cough, hemoptysis (coughing blood), shortness of breath, and hoarseness are typical. Paraneoplastic syndromes like SIADH (hyponatremia), Cushing’s syndrome, or Lambert-Eaton syndrome may precede radiologic findings. Clubbing of the fingers and fatigue are also late but specific signs.
Breast Cancer
Early breast cancer is frequently asymptomatic. Self-detected lumps, nipple retraction, or skin dimpling should be urgently evaluated. Inflammatory breast cancer presents differently: rapid swelling, redness, and warmth of the breast tissue with peau d’orange appearance. Many younger women ignore symptoms due to low perceived risk—leading to delayed diagnoses.
Prostate and Bladder Cancers
Prostate cancer is often insidious, presenting only with urinary hesitancy, nocturia, or back pain due to bone metastases. In men over 60, new-onset lower back pain should raise concern if accompanied by elevated PSA or hematuria. Bladder cancer typically causes painless gross hematuria, which is often misattributed to urinary tract infections.
Gynecological Cancers
Endometrial cancer presents with postmenopausal bleeding—one of the most reliable signs in all of oncology. Ovarian cancer is stealthier: bloating, early satiety, pelvic pain, and increased abdominal girth are common, but nonspecific. These symptoms, especially when recurrent or progressive, require imaging.
Hematologic Cancers (Leukemia, Lymphoma, Myeloma)
These cancers often present with systemic symptoms: persistent fatigue, night sweats, unexplained fever, and weight loss. Lymphadenopathy (non-tender, firm, fixed nodes), recurrent infections, or unexplained bruising should prompt immediate bloodwork and bone marrow evaluation.
When Is a Symptom ‘Definitely’ Cancer: Diagnostic Thresholds in 2025
A central challenge in oncology is balancing vigilance with prudence. Not every back pain is bone metastasis. Not every fever is lymphoma. In 2025, oncologists operate within diagnostic probability frameworks—algorithms that integrate symptom clusters, lab abnormalities, imaging results, and AI-supported risk models to triage patients.
For example, a 65-year-old with weight loss, anemia, and elevated LDH may not yet have visible cancer on a CT scan, but the statistical probability of malignancy exceeds 50%. In such cases, full-body PET-CT or bone marrow biopsy is often justified.
Key Symptom Clusters That Cross Diagnostic Thresholds:
| Symptom Cluster | Likely Evaluation Triggered |
|---|---|
| Unintentional weight loss + anemia + elevated platelets | GI workup: colonoscopy, gastroscopy, CT abdomen |
| Chronic cough + hemoptysis + chest pain | Chest CT, sputum cytology, bronchoscopy |
| Pelvic pain + bloating + early satiety | Pelvic ultrasound, CA-125, CT pelvis |
| Persistent fever + night sweats + lymphadenopathy | CBC, LDH, ESR, PET-CT, possible lymph node biopsy |
| Postmenopausal bleeding | Transvaginal ultrasound, endometrial biopsy |
Modern cancer diagnostics increasingly rely on “3 strikes” logic:
- 1 vague symptom may be ignored
- 2 symptoms in combination should prompt screening
- 3 or more high-risk signs justify full oncologic workup
Additionally, cancer “signatures” like paraneoplastic syndromes now trigger accelerated workflows. For instance, SIADH or dermatomyositis in an adult with no other cause are now considered probable cancer indicators unless proven otherwise.
Finally, the role of biomarkers and liquid biopsy is expanding. In certain cases (e.g., elevated CEA, CA-19-9, or circulating tumor DNA), a lab result alone may push the diagnostic probability high enough to justify targeted imaging even in the absence of symptoms.
Unexplained Weight Loss, Pain, and Fatigue: What They Mean in 2025
In oncology, weight loss, chronic pain, and fatigue have moved beyond vague complaints. In 2025, they are statistically powerful markers—especially when persistent and unexplained by other diseases. These are no longer “soft signs,” particularly when they occur in individuals over 40.
Unintentional Weight Loss
Defined as >5% of body weight lost over 6–12 months, it remains one of the most powerful red flags. In cancer patients, it is often due to:
- Tumor-induced cachexia (via cytokines like TNF-α, IL-6)
- Increased metabolic demand of aggressive tumors
- Malabsorption (GI cancers)
- Mechanical issues (e.g., difficulty swallowing with esophageal cancer)
Weight loss should prompt a comprehensive workup if accompanied by:
- Night sweats or fevers
- Palpable lymph nodes
- Laboratory abnormalities (e.g. elevated ESR or CRP)
Chronic Pain
Cancer-related pain tends to be progressive, unresponsive to over-the-counter analgesics, and localized. Some classic examples:
- Bone metastases from prostate, breast, or lung cancers → deep, dull pain, often nocturnal
- Pancreatic cancer → upper abdominal pain radiating to the back
- Brain tumors → morning headaches, worse with Valsalva, sometimes with nausea
Pain that persists beyond 3–4 weeks and lacks a clear musculoskeletal origin warrants imaging.
Cancer-Related Fatigue
Unlike everyday tiredness, cancer fatigue is non-relieving, overwhelming, and often disproportionate to activity level. It’s especially concerning when accompanied by:
- Dyspnea on exertion
- New anemia or thrombocytopenia
- Depression or cognitive slowing
In modern diagnostic frameworks, fatigue + weight loss + elevated inflammatory markers (CRP/ESR) is a triad requiring escalation—often to whole-body imaging or PET-CT.
What Blood Tests to Request If You Suspect Cancer
While no single blood test confirms cancer, certain panels, when interpreted together, increase suspicion dramatically. The goal is not to diagnose, but to raise or lower the pre-test probability of malignancy, guiding further investigation.
Standard First-Line Panel (When Symptoms Are Unexplained):
| Test | Why It’s Used |
|---|---|
| CBC with differential | Detect anemia, leukopenia, thrombocytopenia |
| ESR and CRP | Inflammatory markers often elevated in malignancy |
| LDH | Elevated in many aggressive cancers and lymphomas |
| Liver enzymes (AST/ALT, ALP) | Detect liver involvement or bone metastases |
| Creatinine / GFR | Baseline for nephrotoxicity, also flags renal tumors |
| Ferritin | Elevated in inflammation, some cancers (e.g. lymphoma) |
| CEA / CA-19-9 / CA-125 | Tumor markers depending on clinical suspicion |
Advanced Markers (Case-Dependent):
- Beta-2 microglobulin: Useful in lymphomas and multiple myeloma.
- PSA: In all men >50 with urinary symptoms or back pain.
- Serum protein electrophoresis (SPEP): Detects M-protein in multiple myeloma.
- AFP / hCG / LDH: In younger males, to rule out testicular cancer.
- Circulating tumor DNA (ctDNA): Becoming more common in private centers for early detection.
In 2025, integration of these results via oncology AI-assistants is common in hospitals. Risk scoring models combine lab data with age, symptoms, family history, and imaging to suggest next diagnostic steps.
The Timeline of Cancer Diagnosis in 2025: From First Symptom to Final Confirmation
Contrary to popular belief, most cancers are not diagnosed at first visit—even in developed systems. In 2025, real-world diagnostic timelines for cancer vary depending on tumor type, symptom clarity, and access to testing.
Median Time to Diagnosis (2025 Data):
| Cancer Type | Time from First Symptom to Diagnosis |
|---|---|
| Pancreatic | 4–7 months |
| Colorectal | 5–12 months |
| Lung (non-small cell) | 3–8 months |
| Breast | 1–3 months (often faster due to screening) |
| Lymphoma | 2–6 months |
| Kidney | 6–9 months |
These delays often occur because early symptoms are nonspecific—fatigue, pain, cough, anemia—and attributed to benign causes (stress, infection, IBS, etc.).
What accelerates diagnosis:
- Proactive doctors ordering imaging early
- Abnormal blood tests with no clear cause
- Patients advocating for further testing
- History of prior malignancy or genetic risk
What delays diagnosis:
- Young age of patient (bias against cancer workup)
- Attribution of symptoms to lifestyle
- Limited access to specialists or imaging
- Reassuring but incomplete blood tests (e.g., normal CBC in lymphoma)
In 2025, AI-enhanced EHR systems in advanced clinics flag “cancer-risk constellations” (e.g., anemia + fatigue + high CRP) and prompt escalation automatically—though not yet universal.
Common Misdiagnoses That Delay Cancer Detection
In the modern clinical setting, many cancers present with signs mimicking benign diseases. Awareness of these patterns helps avoid critical delays.
Most frequent misdiagnosed presentations:
- Irritable Bowel Syndrome (IBS)
— Can mimic colorectal cancer when bloating and diarrhea dominate.
— True red flag: new-onset symptoms after age 50 or iron-deficiency anemia. - Depression or Chronic Fatigue Syndrome
— Lymphoma, leukemia, pancreatic cancer, or brain tumors can present first as apathy, cognitive fog, or “burnout.”
— If fatigue worsens despite antidepressants or persists >3 months, labs + imaging are warranted. - Recurrent Infections
— Seen in leukemia and multiple myeloma: ear infections, sinusitis, UTIs.
— Red flag: abnormal CBC or immune suppression labs. - Musculoskeletal Pain or Sciatica
— Metastatic prostate, breast, or lung cancer often localize in the spine.
— Red flag: pain that worsens at night, does not improve with movement, or appears without injury. - Acid Reflux / Gastritis
— Early gastric or esophageal cancers present with subtle dyspepsia.
— Red flag: new symptoms after age 50 or progressive weight loss.
Misdiagnosis isn’t always due to negligence—it often reflects the silent, “chameleon” nature of early cancer. In 2025, the rule is: “If symptoms don’t improve in 4–6 weeks despite treatment, escalate.”
When Symptoms Don’t Match the Tests: Normal Bloodwork in Cancer
One of the most dangerous myths in medicine is: “If your blood tests are fine, you’re fine.” In reality, many cancers do not alter basic labs early on. This is especially true in the absence of systemic inflammation or organ damage.
Most misleading lab panels:
- Normal CBC despite presence of early lymphoma, kidney cancer, or brain tumors
- Normal liver enzymes even with small liver metastases
- Normal ESR/CRP in some solid tumors until late stages
- Normal LDH in indolent lymphomas or non-metastatic disease
This is why in 2025, the diagnostic focus is shifting from isolated labs to pattern-based risk models. For example:
- Patient A: Normal labs + unexplained weight loss + persistent cough → CT chest
- Patient B: Normal labs + postmenopausal bleeding → urgent transvaginal ultrasound + biopsy
Rule of thumb: Normal bloodwork is not a green light if symptoms are persistent, progressive, or focal. Imaging or tissue sampling is the only definitive next step.
In many regions, AI-assisted triage tools now suggest scans or biopsies even when labs are “reassuring” but the symptom pattern fits early cancer signatures.
Tumor Markers: How Much Can You Rely on Them in 2025?
Tumor markers have been both overused and misunderstood for decades. In 2025, oncology consensus has matured: they are useful tools, but only when applied correctly and never in isolation.
What tumor markers are not:
- They do not confirm or exclude cancer alone.
- They are not effective for screening in the general population.
- They can be elevated for non-cancer reasons: inflammation, liver disease, smoking, etc.
When tumor markers do help:
| Marker | Used in | Best Use Case |
|---|---|---|
| CEA | Colorectal, pancreatic, breast | Monitoring known cancer, detecting recurrence |
| CA-125 | Ovarian cancer | Treatment response, recurrence detection |
| CA 19-9 | Pancreatic cancer | Post-diagnosis monitoring |
| PSA | Prostate cancer | Screening in men >50 with risk factors |
| AFP | Liver, testicular cancer | Diagnosis + monitoring |
| Beta-hCG | Germ cell tumors | Diagnosis + follow-up |
| LDH | Lymphoma, testicular cancer | Aggressiveness and tumor burden indicator |
In advanced cancer care centers, tumor markers are increasingly used in composite algorithms — alongside imaging and liquid biopsies — not as solo tools.
For example, an elevated CEA in a post-surgical colon cancer patient might signal early recurrence months before imaging confirms it. But an elevated CEA in a smoker without symptoms may be meaningless.
Symptoms That Mimic Other Diseases — but Are Actually Cancer
Cancer rarely knocks on the door with a clear label. In most cases, it shows up in disguise, borrowing the appearance of common illnesses. This is why misdiagnosis or delayed diagnosis remains a significant issue worldwide.
Here are a few classic scenarios:
1. Persistent Heartburn = Esophageal or Gastric Cancer
Patients are treated for months with antacids or PPIs. The real cause? A growing tumor in the esophagus or stomach.
Red flags: Progressive dysphagia, weight loss, anemia, vomiting blood (hematemesis).
2. Chronic Cough = Lung Cancer
Especially in non-smokers, persistent cough is dismissed as post-viral or asthma. In reality, adenocarcinoma of the lung is now one of the most common non-smoker cancers.
Red flags: Cough lasting >6 weeks, coughing up blood, fatigue, hoarseness.
3. Menstrual Irregularities = Cervical or Endometrial Cancer
Many young women are told it’s just hormonal imbalance. If bleeding is unusual, post-coital, or persistent, a pelvic exam and ultrasound are essential.
4. Back Pain = Bone Metastasis
Especially when pain is worse at night or doesn’t respond to rest. Breast, prostate, lung, and kidney cancers often metastasize to bone early.
5. Irritable Bowel Syndrome = Colon or Ovarian Cancer
A common misdiagnosis in women. If symptoms start after age 40–45, insist on imaging and blood work.
Red flags: Bloating, early satiety, family history, unintended weight loss.
The general rule in 2025: if symptoms persist beyond 4–6 weeks, even with treatment, they need deeper investigation—imaging, biopsy, or referral.
How 2025 AI Models Predict Cancer Risk Early
With access to massive EHR datasets, machine learning models in 2025 can now detect subtle cancer patterns long before a human clinician would consider cancer.
How it works:
AI models are trained on:
- Longitudinal health records (labs, symptoms, prescriptions)
- Imaging reports and pathology history
- Social determinants (smoking, location, family history)
When a patient presents with common but “suspicious combinations”, the model may flag for early imaging or referral.
Example:
- A 52-year-old female with new-onset bloating + fatigue + slightly raised CA-125
→ flagged for transvaginal ultrasound and oncology referral
→ detected Stage I ovarian cancer
Another case:
- A 38-year-old man with normal labs but persistent night sweats and subtle weight loss
→ AI model suggests PET-CT
→ found early-stage Hodgkin’s lymphoma
These systems aren’t replacing doctors — but in clinics where they are implemented, diagnostic delays have dropped by 18–22% in the last two years.
In the next decade, we expect AI-driven “pre-diagnostic triage” to become standard protocol, especially in large health systems.
Blood Tests That Actually Save Lives
While many cancer cases aren’t caught by basic lab panels, certain targeted blood tests can lead to life-saving early detection — if used wisely.
Cases where blood work can reveal cancer early:
1. Multiple Myeloma
- Abnormal labs: Elevated total protein, low albumin, anemia, high calcium
- Key tests: SPEP (serum protein electrophoresis), free light chains, beta-2 microglobulin
These markers often trigger early suspicion before bone lesions even appear.
2. Chronic Myeloid Leukemia (CML)
- Abnormal labs: Very high white blood cell (WBC) count, often found on routine CBC
- Confirmatory tests: BCR-ABL gene via PCR
3. Pancreatic or Colon Cancer
- Clue: Unexplained anemia + iron deficiency + occult blood in stool
- Follow-up: Colonoscopy or abdominal CT
Comparative table of life-saving blood markers:
| Test | Cancer Type | When It Helps |
|---|---|---|
| PSA | Prostate | Early detection in men >50 |
| FIT test | Colon | Detects blood in stool |
| AFP / Beta-hCG | Testicular, liver | Diagnostic + follow-up |
| Bence-Jones protein | Myeloma | Part of urine test for light chains |
| CBC + peripheral smear | Leukemias, lymphomas | Detects blast cells, anemia, cytopenias |
These tests are only effective when paired with clinical judgment and follow-up imaging.
An abnormal test isn’t a death sentence. But ignoring early flags can be.
Full-Body Scans: When They Help, When They Don’t
The rise of executive checkups and private CT/MRI clinics has made full-body scanning a trend — often marketed as a “preventive” tool. But in clinical oncology, we take a more measured view.
When full-body scans are justified:
- High-risk genetic carriers (e.g. BRCA1/2, Lynch syndrome)
- Patients with multiple vague symptoms + family history
- Unknown primary cancer (CUP) when metastatic sites are detected
- Follow-up in survivors with elevated tumor markers
When scans are more harm than help:
- Asymptomatic patients with no history or risk
- Scans every year “just in case” → leads to overdiagnosis
- Incidentalomas: benign nodules, cysts, scars → anxiety + unnecessary biopsies
Scans are like searchlights: use them when you know where to look. Random scanning increases false positives, radiation exposure, and cost — with little survival benefit.
Best practice in 2025:
- Use imaging in algorithmic, symptom-guided fashion
- Combine AI triage, lab markers, family history before full-body decision
FAQ
Can I have cancer if all my blood tests are normal?
Yes. Many early-stage cancers don’t affect routine blood tests like CBC or metabolic panels. Some solid tumors may grow silently. If symptoms persist despite normal labs, imaging or biopsy may still be needed.
My hemoglobin is low, but I’m not bleeding — could it be cancer?
Low hemoglobin without obvious blood loss can signal gastrointestinal, kidney, or bone marrow cancers. Further testing like colonoscopy, abdominal ultrasound, or bone marrow biopsy may be required.
I have night sweats and fatigue — is that always lymphoma?
Not always. But when combined with unexplained weight loss or swollen lymph nodes, it becomes suspicious. A physical exam, CBC, LDH, and imaging are the next steps.
How reliable is ESR or CRP in detecting cancer?
They’re nonspecific. Elevated ESR/CRP indicate inflammation, which may be from infection, autoimmune disease, or cancer. They support the diagnosis but don’t confirm it.
Should I worry about a slightly high CEA level?
Mild CEA elevations can occur from smoking, inflammation, or benign polyps. Values >5–6 ng/mL in non-smokers, especially if rising, are more concerning and need workup.
What cancers cause high platelets (thrombocytosis)?
Lung, colon, and ovarian cancers are known to trigger reactive thrombocytosis. Persistently high platelet counts without inflammation or iron deficiency require cancer screening.
Can lung cancer exist with a normal chest X-ray?
Yes. X-rays can miss early or small tumors, especially in the periphery. If there’s suspicion, a chest CT is more sensitive and should be ordered.
I have a swollen lymph node for 2 months — is it cancer?
Duration, firmness, and location matter. Nodes >2 cm, hard, fixed, and painless (especially in the neck, armpit, or groin) should be biopsied.
My LDH is high — what does it mean?
High LDH may indicate cell turnover or tissue damage. It’s often elevated in lymphomas, leukemias, and metastatic cancer. It’s not diagnostic alone but important in staging and prognosis.
Is anemia always a warning sign of cancer?
No. Anemia is common and can result from iron deficiency, B12 deficiency, or chronic disease. But if it’s unexplained or doesn’t improve, cancer workup is reasonable.
Do normal tumor markers rule out cancer?
No. Tumor markers like CA-125, CEA, or PSA may be normal in early-stage disease or in tumors that don’t produce markers. Diagnosis must be based on multiple factors.
My WBC count is a bit low — should I worry about leukemia?
Mild leukopenia may occur from viral infections or medications. But if it’s persistent, or paired with fatigue, bruising, or infections, bone marrow tests may be needed.
Can cancer start without any symptoms?
Yes. Especially in pancreas, ovary, kidney, or prostate. This is why routine screening and attention to subtle changes (appetite, energy, weight) are critical.
What’s the fastest way to confirm or rule out cancer?
Depends on the location. Biopsy remains the gold standard. Imaging (CT, PET, MRI) can guide diagnosis. Blood tests help, but never confirm alone.
Should I panic if I find a lump?
No panic — but don’t ignore it. Most lumps are benign, but those that grow, are firm, fixed, or painless should be examined, scanned, or biopsied.