Re-irradiation for Endometrial Cancer: Key Insights & Outcomes
Introduction to Endometrial Cancer
If you’re reading this, there’s a good chance you or someone close to you is navigating the challenges of endometrial cancer—perhaps even facing the tough prospect of recurrence. You might be wondering: Why does this cancer come back? What are the options when it does? And where does something like re-irradiation even fit in? All excellent questions—and to answer them meaningfully, we first need to understand what endometrial cancer is, how it behaves, and what we typically do about it.
What exactly is endometrial cancer?
Endometrial cancer originates in the lining of the uterus—the endometrium. It’s the most common gynecologic malignancy in developed countries, with incidence rates steadily increasing due to shifting demographics and risk profiles. It often strikes postmenopausal women, but not exclusively. And while the majority of cases are diagnosed at an early stage—often due to the telltale symptom of abnormal vaginal bleeding—that doesn’t mean the story always ends there.
There are two major types of endometrial cancer:
- Type I, which is usually hormone-driven, tends to be lower grade, and has a better prognosis.
- Type II, which is non-hormonal, more aggressive, and often diagnosed at a later stage.
This distinction isn’t just academic. It influences everything from treatment planning to the risk of recurrence.
What are the usual treatments?
For most patients, the treatment path begins with surgery—typically a total hysterectomy with removal of the fallopian tubes and ovaries. Depending on the stage and histological features of the tumor, adjuvant therapies like radiation or chemotherapy may follow. Radiation therapy, in particular, plays a critical role in local control. For early-stage, high-intermediate risk patients, vaginal brachytherapy or external beam radiation can significantly reduce the chance of recurrence.
But here’s the uncomfortable truth: recurrence still happens.
Why does endometrial cancer recur?
Even after what appears to be a curative treatment, cancer can return. In endometrial cancer, recurrence typically shows up in one of three ways:
- Local recurrence – in the vaginal cuff or pelvis.
- Regional recurrence – involving nearby lymph nodes.
- Distant metastases – to the lungs, liver, or elsewhere.
The risk of recurrence depends on multiple factors—tumor grade, lymphovascular invasion, depth of myometrial invasion, and whether lymph nodes were involved. Type II histologies like serous or clear cell carcinomas, for instance, carry a notoriously higher risk of spread and relapse.
This leads us to a critical clinical fork in the road: what do you do when cancer returns—especially in a site that was previously irradiated?
Why is re-irradiation even being discussed?
Radiation therapy is a double-edged sword. It’s highly effective at controlling local disease, but it also leaves a lasting footprint on the tissues it targets. That means if a recurrence happens in an area that’s already received its “maximum safe dose,” the conventional wisdom used to be: you’re out of luck. Surgery, if feasible, was usually the only recourse.
But advances in imaging, radiation physics, and biologically informed planning have opened new doors. Re-irradiation—once considered too risky—is now being carefully reconsidered in select cases. And for some patients, it offers a second chance at durable control, or even cure.
Before we dive into the nuances of when and how re-irradiation can be applied (we’ll get to that in Part 2), it’s worth anchoring ourselves in the broader context: endometrial cancer isn’t monolithic. It’s a biologically diverse, clinically complex disease that demands individualized care. And understanding the terrain—both of the disease and the treatments we deploy—is the first step toward making informed, confident choices when the path gets harder.
This isn’t a frontline treatment, but when options run thin, it can become a critical consideration — similar to how Dense Dose Chemotherapy is used in aggressive or relapsing breast cancer cases.
So if you’re wondering: Is it even possible to treat a relapse after radiation?—the answer is yes, in some cases, and with nuance. But that nuance is exactly why this conversation matters. Let’s keep going.
Understanding Re-irradiation
Let’s cut to the chase: re-irradiation is not a default move. It’s a calculated decision, one that walks a tightrope between potential benefit and possible harm. But before we weigh its pros and cons, we need to get very clear about what it is—and what it isn’t.
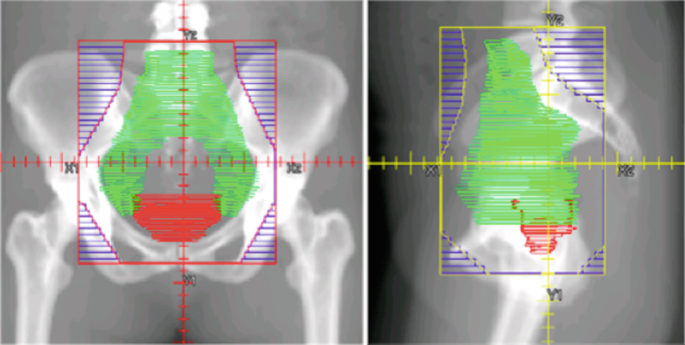
So, what exactly is re-irradiation?
In simple terms, re-irradiation means giving radiation therapy to a part of the body that has already received it. But don’t let the simplicity of that definition fool you. In practice, it’s a technically demanding and medically nuanced maneuver. You’re not just repeating what you did the first time. You’re working with a landscape that’s already been scarred—sometimes subtly, sometimes dramatically—by prior radiation. The tissues have changed. The tumor may be harder to reach or behaving differently. And the margin for error? Considerably narrower.
You might be wondering: Why would anyone take that risk? The short answer is because in some cases, the alternative is worse.
When a recurrence happens in a previously irradiated field—say, the pelvic sidewall or vaginal cuff—the standard treatment options shrink dramatically. Surgery may be technically impossible or medically unsafe. Systemic therapies, like chemotherapy or hormonal agents, may be considered, but they’re often less effective in purely local disease. That’s where re-irradiation enters the conversation: not as a first-line hero, but as a specialized tool for very specific, often high-stakes scenarios.
Has re-irradiation always been on the table?
Not really. Historically, re-irradiation was largely dismissed as too dangerous. Radiation’s effect on normal tissues is cumulative, and the pelvis houses structures—bladder, rectum, bowel—that don’t tolerate repeat radiation well. The fear was, and still is to some extent, that re-treating an already-irradiated area could lead to catastrophic complications: bowel perforations, fistulas, radiation necrosis.
But the story has evolved. Technological advances have revolutionized how we think about radiation. Modern tools like intensity-modulated radiation therapy (IMRT), image-guided radiotherapy (IGRT), and high-dose-rate (HDR) brachytherapy allow for far more precise targeting. We’re no longer just “radiating the pelvis”—we’re contouring tight volumes, sculpting dose, and using imaging in real time to avoid critical structures. In effect, we’ve gone from using a paint roller to a fine-tip brush.
And let’s not forget the role of biology. As we gain more insight into individual tumor behaviors—how aggressively they grow, how they respond to radiation, how they differ genetically—we can start to tailor re-irradiation more intelligently. Some patients may have slow-growing recurrences in well-defined areas. Others might have more diffuse disease. These differences matter, and they shape the risk-benefit equation.
Is re-irradiation for everyone?
No. And here’s where the nuance comes in. Re-irradiation isn’t a one-size-fits-all option. It’s not something you offer casually. The decision depends on:
- Where the recurrence is (and how close it is to previously treated organs).
- How long it’s been since the last radiation (tissues need time to partially recover).
- What techniques were used before (older methods were less precise).
- The patient’s current health and treatment goals (curative vs. palliative intent).
In some cases, re-irradiation may be the only curative option left on the table. In others, it might offer local control and symptom relief in a palliative setting. And in a few, it might simply not be worth the risk.
One question we hear a lot is: Can you “undo” the previous radiation damage to make re-irradiation safer?Unfortunately, not really. Once tissues are irradiated, they change permanently at the cellular level. But the body is remarkably adaptable, and with time, some degree of healing occurs. This recovery—along with modern dose modeling and precise delivery—forms the basis for calculating how much additional radiation can be given without crossing into danger zones.
Are there any success stories?
Absolutely. And we’ll get into specific case studies and data in later sections, but for now, it’s worth knowing that re-irradiation isn’t just theoretical. It’s been done. Successfully. With curative outcomes. In fact, several centers around the world have published encouraging results using re-irradiation in select patients with recurrent gynecologic cancers, including endometrial.
But success requires planning, patience, and often, a multi-disciplinary approach. Radiation oncologists don’t make these decisions alone. They work alongside surgical oncologists, medical oncologists, radiologists, and sometimes even reconstructive surgeons to design a treatment strategy that respects both the disease and the host.
In short, re-irradiation is no longer a forbidden territory—it’s a frontier. And like all frontiers, it demands expertise, innovation, and a deep respect for the terrain.
Coming up, we’ll dive deeper into when re-irradiation actually gets considered: what the clinical triggers are, how patient selection works, and what kind of factors tip the scales. Because if there’s one thing that’s clear, it’s this—when it comes to re-irradiation in endometrial cancer, the decision-making is just as important as the delivery.
Indications for Re-irradiation in Endometrial Cancer
By now, you’ve probably gathered that re-irradiation isn’t something you stumble into. It’s not offered on a whim or used just because “nothing else worked.” So how does a patient even get to the point where re-irradiation becomes a serious consideration? Who qualifies—and perhaps more importantly, who doesn’t?
Let’s start with a common misconception: that all recurrences are created equal. They’re not. The what, where, and whenof a recurrence profoundly influence whether re-irradiation is even on the table.
So who exactly is a candidate?
The ideal candidate for re-irradiation is someone with a localized recurrence—meaning the tumor is confined to a previously treated area and hasn’t spread distantly—and has already exhausted safer alternatives. The recurrence must be in a location that can be precisely targeted without placing nearby organs at unacceptable risk. Most often, this involves the vaginal cuff, pelvic wall, or regional lymph nodes.
But that’s just the start. The radiation oncologist will also consider several critical variables:
- Time since initial radiation: This matters because tissues need time to repair. A recurrence just a few months after initial treatment suggests radioresistant disease—and very little time for healing. But if years have passed, the tissues may be more tolerant of another, carefully calibrated dose.
- Previous dose and technique: Knowing exactly how much radiation was delivered the first time, and how it was delivered, is essential. Was it external beam? Brachytherapy? Were advanced planning methods used to spare organs at risk? Many centers now keep detailed dose-volume histograms from prior treatments, which can be a goldmine in planning a second course safely.
- Patient health and goals: Not every patient will pursue curative treatment at recurrence. Age, comorbidities, performance status—all of these influence whether aggressive local therapy is appropriate. But for a patient in good general health, who is otherwise disease-free, a small local recurrence may well be something worth treating with curative intent.
- Tumor biology: Histology and grade matter here, too. A slow-growing, low-grade recurrence is fundamentally different from a fast-moving, high-grade one. Type I endometrioid tumors often behave differently than type II serous or clear cell types, which are known for their aggressiveness and higher relapse rates. A well-differentiated tumor in a favorable location is much more likely to respond safely to re-irradiation than a diffuse, poorly differentiated one wrapped around vital structures.
At this point, you might ask: How do doctors actually decide whether to proceed? And the answer is: through layered, case-by-case deliberation. A tumor board discussion is typical—radiation oncologists, gynecologic oncologists, radiologists, and sometimes pathologists and palliative care experts all weigh in. Imaging is reviewed meticulously. Risks are modeled. Patient priorities are factored in. And if the consensus is that re-irradiation could meaningfully extend survival or improve quality of life, and the risks are considered manageable, it moves forward.
But let’s be clear—there are just as many times when re-irradiation is ruled out.
When is re-irradiation not recommended?
Several red flags can make re-irradiation an unacceptable risk:
- If the recurrence involves the bowel or bladder directly, and these organs already received high doses previously, the risk of severe toxicity (fistulas, necrosis) may outweigh any potential benefit.
- If the recurrence is multifocal or shows distant spread, then the role of local therapy becomes questionable—why target a single spot when the disease is everywhere?
- And if the patient has poor functional status or significant comorbidities that preclude tolerating further treatment, even the most expertly delivered re-irradiation can do more harm than good.
This is the kind of terrain where medicine becomes less of a science and more of an art. You’re not following a rigid protocol; you’re integrating evidence, clinical acumen, patient values, and logistical reality. For example, a woman in her 70s with a 2 cm isolated vaginal cuff recurrence, three years after pelvic radiation, otherwise healthy and strongly motivated to pursue curative options? She might be a perfect candidate. But change any one of those variables, and the risk-benefit ratio could shift dramatically.
Are there alternatives?
Absolutely. And they’re always part of the discussion. Surgery may still be an option in selected patients, especially if it’s technically feasible and the patient is fit. Exenteration—a radical procedure removing pelvic organs—is sometimes considered, though it comes with major morbidity. Chemotherapy and hormonal therapy may be used either as primary treatment or in conjunction with localized options. And for some patients, especially those with indolent tumors or limited life expectancy, best supportive care may be the most compassionate choice.
Re-irradiation doesn’t exist in a vacuum. It’s one option in a toolbox, and it only makes sense when the clinical context aligns. But when the stars do align—when disease is limited, the patient is well, and the anatomy allows—re-irradiation can offer a real shot at durable control. In some cases, even cure.
So if you’ve been told re-irradiation is “off the table,” it’s worth asking: Why, exactly? And if you’ve been told it’s possible, you should also ask: How, and with what risks? Because the answers to those questions aren’t generic—they’re yours. Personalized, contextual, and crucial.
If you’re also navigating healthcare coverage and recovery expectations, VA Ratings for Cancer Remission sheds light on how post-treatment support works in real life.
In the next section, we’ll move from who to how—exploring the various techniques used to safely and effectively re-irradiate in endometrial cancer. From brachytherapy to stereotactic radiation, we’ll unpack the technologies and tactics that make this once-taboo approach not just possible, but promising.
Re-irradiation Techniques and Modalities
By now, you’re likely wondering: If re-irradiation is such a tightrope act, how do clinicians actually do it? What tools exist to make it not just tolerable, but effective? This is where the conversation gets both technical and exciting—because re-irradiation isn’t a single procedure. It’s a toolkit of evolving techniques, each with its own philosophy, precision, and purpose. Let’s take a tour through the main modalities currently in play for re-irradiating endometrial cancer, and what makes each one either promising or perilous.
Brachytherapy: The Inside Job
Let’s start with a favorite in gynecologic oncology—brachytherapy, which delivers radiation from inside the body, directly into or near the tumor. Think of it as the “sniper” of radiation therapies. Instead of blasting a broad field from outside the body, brachytherapy uses applicators placed inside the vagina, uterine remnant, or pelvic tissues to deliver tightly confined doses to a precise location.
When a recurrence happens in the vaginal cuff, for example, and especially if the patient only had external beam radiation during her initial treatment, high-dose-rate (HDR) brachytherapy can be a game-changer. Why? Because it allows clinicians to deliver a therapeutic dose to the tumor bed while sparing nearby organs like the bladder and rectum from significant exposure. Even in previously irradiated fields, the ultra-localized nature of brachytherapy allows for retreatment—albeit with extremely careful planning.
But there’s a catch: it only works if the disease is confined. If a tumor is bulky, extends deep into pelvic tissues, or has infiltrated organs, brachytherapy’s precision becomes a limitation. You can’t “brachy” your way through large or irregularly shaped recurrences. In those cases, external beam radiation or more advanced options may be required.
External Beam Radiation Therapy (EBRT): The Old Workhorse, Evolved
The phrase “external beam radiation” might conjure an image of a clunky, high-powered machine indiscriminately blasting the pelvis. That was yesterday. Today’s EBRT, especially when using Intensity-Modulated Radiation Therapy (IMRT) or Volumetric Modulated Arc Therapy (VMAT), is a far cry from the blunt instruments of the past.
IMRT allows clinicians to modulate the dose in real time, shaping radiation beams to match the contours of the target while minimizing exposure to critical structures. In a re-irradiation context, that’s everything. You’re not just trying to hit the tumor—you’re trying to not hit the bowel, bladder, and sacral plexus, which may have already soaked up significant radiation during the first round of treatment.
The other key evolution is imaging. Image-guided radiation therapy (IGRT), which involves daily imaging (CT, cone-beam, or MRI) before or during treatment, ensures that any anatomical shifts—bladder filling, rectal distention, weight changes—don’t throw off the plan. That level of precision can mean the difference between durable tumor control and a treatment-related complication.
Still, EBRT has its limits. If the recurrence is nestled right against previously damaged tissues—or if a patient’s organs have already declared their intolerance—external beam may be too risky. That’s where the next generation of techniques comes into play.
Stereotactic Body Radiotherapy (SBRT): The Precision Bomb
Now we’re getting futuristic. Stereotactic Body Radiotherapy (SBRT) delivers very high doses of radiation in a small number of treatments (sometimes even a single fraction), with millimeter-level accuracy. Picture a surgical strike rather than a military campaign.
SBRT’s hallmark is its steep dose gradient—the radiation drops off sharply outside the target area, which is ideal in re-irradiation scenarios where the margin for error is vanishingly thin. It’s particularly useful for nodal recurrences in the pelvis or para-aortic area, where precise, ablative doses can be used without re-treating the whole field.
But SBRT isn’t for everyone. It requires high-end imaging, rock-solid immobilization, and extremely detailed planning. It’s best suited for small-volume, well-defined recurrences in patients who can lie still for longer periods and who have sufficient spacing between the target and critical structures.
Proton and Carbon Ion Therapy: The Frontier Tools
Let’s talk about proton therapy. Unlike traditional X-rays, which deposit energy along their entire path, protons release most of their energy at a single depth—called the Bragg peak—and then stop. That means less radiation to surrounding tissues. In theory, this makes protons ideal for re-irradiating areas adjacent to sensitive organs.
There’s a caveat, though: proton therapy is expensive, not widely available, and requires specialized expertise. While promising, it’s not yet a mainstay in gynecologic re-irradiation protocols—but it’s being studied actively, and some centers have reported favorable early outcomes.
Even more exotic is carbon ion therapy, which offers even higher precision and potentially greater biological effectiveness than protons. It’s mostly available in Japan and select European centers, and while the data is still emerging, it could one day play a significant role for patients with particularly resistant or deeply seated tumors.
Intraoperative Radiation Therapy (IORT): Hitting While the Hood’s Up
IORT is exactly what it sounds like—radiation delivered during surgery, right to the exposed tumor bed. Imagine a patient with a pelvic recurrence undergoing tumor resection. After the tumor is removed, radiation is applied directly to the area at risk before the surgeon closes up.
This has a few advantages. You can move organs like the bowel out of the way, physically shield structures you want to spare, and apply a high dose exactly where it’s needed. But it’s not widely available, requires logistical coordination between surgery and radiation teams, and is only feasible in select cases.
So where does all this leave us?
Re-irradiation in endometrial cancer is no longer an exercise in wishful thinking. It’s a technically rich, clinically rigorous field that’s evolving rapidly. Whether through brachytherapy’s intimacy, IMRT’s sculpting finesse, or SBRT’s surgical accuracy, we now have the means to deliver second courses of radiation with intent and precision.
But—and this is crucial—no technique is universally superior. The “best” approach depends on the patient’s anatomy, tumor characteristics, prior treatment history, and overall goals. There are trade-offs with every modality, and choosing the right one requires not just technological prowess, but judgment, collaboration, and a thorough understanding of what’s at stake.
In the next section, we’ll look at outcomes. Does all this effort pay off? What do the numbers say about survival, local control, and—perhaps most importantly—quality of life after re-irradiation? Spoiler: the answers are nuanced, but they matter deeply.
Clinical Outcomes and Efficacy
Now that we’ve covered the “how,” let’s turn to the question that really drives every clinical decision: Does it work? In other words, when re-irradiation is used in endometrial cancer, what kinds of results can we expect? Are we buying time? Buying quality of life? Chasing a cure? These are not just abstract queries—they’re the exact conversations taking place in tumor boards, consult rooms, and patient families every day.
The short answer? Re-irradiation can work—sometimes impressively so—but the outcomes are intimately tied to the context. And as with much in oncology, the nuance is where the truth lives.
Let’s start with local control. What are the odds of keeping the recurrence in check?
In the right setting—think isolated vaginal cuff recurrences, especially if the patient didn’t receive brachytherapy the first time—local control rates can be surprisingly high. Several retrospective studies and institutional series report 2- to 5-year local control rates in the ballpark of 60% to 80%, especially when brachytherapy is used for small, well-defined recurrences.
That’s not trivial. For patients with no other curative options, the ability to control a localized recurrence without the need for exenteration (a radical, life-altering surgery) is a major win.
But, as you might expect, the story changes with larger tumors, more aggressive histologies, or recurrences involving previously irradiated bowel or bladder. In those cases, the risks of uncontrolled disease and treatment-related toxicity start to weigh more heavily, and local control becomes harder to achieve.
What about survival—does re-irradiation meaningfully extend life?
Here’s where things get even more nuanced. Yes, re-irradiation can improve overall survival in select patients—especially those with isolated recurrences, good performance status, and long disease-free intervals. In such cases, 2- and even 5-year survival rates have been reported between 40% and 70%, depending on the series and patient selection.
But—and this is crucial—it’s not always about curing the disease. Sometimes, re-irradiation is used to buy meaningful time or to convert an urgent, symptomatic situation into a manageable one. A patient with bleeding from a recurrent vaginal lesion, or pelvic pain from a tumor compressing nerves, may find real relief—and restored dignity—from a short course of focused re-treatment.
Survival data must always be interpreted with caution in re-irradiation studies. Most of the literature comes from retrospective analyses, often from single institutions, and usually with carefully selected patients. That’s not to say the numbers are unreliable, but they do reflect a best-case scenario. Real-world outcomes may be less rosy, particularly for patients with comorbidities or less favorable tumor biology.
And what about quality of life? Isn’t that just as important?
Absolutely. For many patients—especially those who’ve already endured one full course of pelvic treatment—the notion of “more radiation” is understandably anxiety-provoking. Fatigue, GI symptoms, sexual dysfunction, and bladder issues may already be part of their daily reality. So any discussion of re-irradiation must squarely confront the potential for further compromise.
Interestingly, in carefully selected cases, re-irradiation doesn’t necessarily worsen quality of life—at least not significantly. That’s particularly true with brachytherapy, where doses are confined, treatment times are short, and complications tend to be localized and manageable. When patients are properly counseled and monitored, many find the side effects tolerable—and some even experience symptom relief.
But this isn’t a one-way street. There are also risks of late toxicity, including:
- Radiation proctitis or cystitis (inflammation of the rectum or bladder),
- Fistulas (abnormal connections between organs),
- Pelvic fibrosis, leading to pain or decreased mobility,
- And, in very rare cases, radiation necrosis—where tissue damage becomes irreversible.
These complications can be devastating, and they often emerge months or even years after treatment. Which is why any clinician who’s been through this enough times will tell you: the decision to re-irradiate must be guided not just by possibility, but by probability, and above all, by perspective.
So, is it worth it?
That’s the million-dollar question, isn’t it? And the answer is: sometimes—powerfully so. When the recurrence is small, localized, and caught early; when the patient is healthy and engaged; and when the team delivering care is experienced and precise—then re-irradiation can offer a second chance. Not just at disease control, but at more life. Better life. Life with less pain, less fear, more agency.
But in other situations, the equation is murkier. A patient with widespread disease, multiple prior treatments, and a limited prognosis may not benefit from another round of radiation. For her, the more ethical, more human choice might be to focus on comfort, stability, and moments that matter.
And that’s okay. That’s medicine done right.
Toxicity and Side Effects
If you’ve made it this far, you already understand that re-irradiation isn’t something offered lightly. It’s not a “second try” in the casual sense—it’s a sophisticated attempt to strike a very precise balance. And here’s the rub: even with our best planning, toxicity is the shadow that always follows re-irradiation. The possibility of hurting something we’re trying to preserve is not just theoretical—it’s real, and sometimes, it’s unavoidable.
So let’s talk about that openly. What kind of side effects are we really dealing with? How often do they happen? Are they manageable—or life-altering?
First, what do we mean by “toxicity”?
In radiation oncology, toxicity refers to the collateral damage—harm to normal tissues that occurs either as an immediate reaction (acute toxicity) or later down the line (late toxicity). Both categories matter. Acute toxicities are often reversible, irritating, and temporary. Late toxicities, on the other hand, can be persistent—or even permanent. And with re-irradiation, the latter is where our focus sharpens.
Radiation to the pelvis—whether initial or repeat—inevitably brushes up against organs that don’t like being brushed at all: the rectum, the bladder, the small bowel, the vaginal canal, nerves, and blood vessels. These tissues have a cumulative memory. Unlike tumors, which we want to kill, normal cells don’t always bounce back with grace after a second hit.
So what does acute toxicity look like?
Acute side effects typically begin during treatment or within a few weeks of its completion. Patients might experience:
- Urinary irritation: urgency, frequency, burning—much like a urinary tract infection, but caused by inflammation rather than bacteria.
- Rectal symptoms: loose stools, mild bleeding, or a sense of urgency.
- Fatigue: an underappreciated but common symptom, driven by both biological and psychological factors.
- Local discomfort: especially with brachytherapy, which can cause vaginal soreness or temporary discharge.
Most of these symptoms are self-limiting and respond well to supportive care—hydration, stool softeners, anti-inflammatories. They rarely cause treatment to be interrupted. But even when they’re transient, they’re not trivial. For a patient who’s already been through one course of radiation, the reappearance of old symptoms can be emotionally triggering. There’s often a psychological toll to going back into that same radiation room and feeling the same sensations.
Late toxicity: what happens months—or years—later?
This is where things get serious. Late toxicities may take six months, a year, or more to manifest, but when they do, they can significantly impact quality of life. And because these effects are often irreversible, they deserve deep respect during the planning process.
Some of the more concerning late toxicities include:
- Radiation cystitis: chronic inflammation of the bladder that can cause bleeding, frequency, and pain. In rare cases, it can progress to hematuria requiring interventions like cauterization or bladder instillation therapy.
- Radiation proctitis: inflammation of the rectal lining that can lead to bleeding, urgency, or strictures. Some patients may need medications, endoscopic therapy, or even surgery to manage complications.
- Fistula formation: perhaps the most feared complication in this context. A fistula is an abnormal connection between two hollow organs—say, between the bladder and the vagina, or the rectum and the uterus. These can lead to incontinence, infection, and significant distress. Surgical repair is possible but not always successful.
- Pelvic fibrosis: over time, radiation can lead to scarring and tightening of soft tissues. This can cause chronic pelvic pain, limited mobility, or vaginal stenosis (narrowing of the vaginal canal), which can interfere with intimacy and examinations.
- Neuropathy: if radiation affects the pelvic nerves, patients can experience numbness, tingling, or even weakness in the lower extremities. It’s rare—but it does happen.
This all sounds terrifying. So why do it?
Because, in some cases, the alternative is worse. Untreated local recurrences can erode into organs, cause uncontrollable bleeding, or compress nerves to the point of immobility. The question isn’t, “Do we risk side effects?” It’s, “Can we offer benefit without overwhelming harm?”
Can toxicity be predicted—or prevented?
To some extent, yes. Radiation oncology is no longer about aiming in the general direction of a tumor and hoping for the best. We have dose constraints for every major organ—limits we don’t cross, based on decades of data and modeling. When planning a re-irradiation course, physicists and physicians work side by side to map previous doses, calculate residual tolerance, and contour new treatment volumes that avoid high-risk zones.
Techniques like IMRT and brachytherapy are especially valuable here. They allow for tighter dose sculpting, steeper fall-off, and more selective sparing of organs. In some cases, devices like vaginal spacers or bladder-filling protocols are used to physically move organs away from the radiation field.
And patient factors matter, too. A healthy, well-nourished patient with good pelvic floor function is more likely to tolerate radiation than someone with diabetes, poor circulation, or pre-existing bowel issues. It’s one more reason why selection is so critical—because the best way to manage toxicity is to avoid setting the wrong patient up for it in the first place.
What’s the bottom line?
Toxicity is not a side note in the re-irradiation story. It’s a central character. But it’s not the villain it once was. With better technology, better planning, and more thoughtful patient selection, we’ve pushed the envelope of what’s possible—and safe.
Still, every patient considering re-irradiation deserves a brutally honest discussion about risks. Not a scare tactic, but a reality check. What could go wrong? How likely is it? What happens if it does? These questions don’t just inform consent—they build trust.
Case Studies and Clinical Trials
By this point, you might be thinking: Okay, I understand the mechanics and the risks—but how does this actually play out in the real world? What happens when a patient says yes to re-irradiation? That’s where case studies come in. These aren’t just academic footnotes—they’re snapshots of decision-making under pressure, of real patients and their outcomes, of how theory gets stress-tested by reality.
So let’s start there—with the stories. Because in medicine, data gives us patterns, but stories give us context. And both are crucial.
What does a successful re-irradiation case look like?
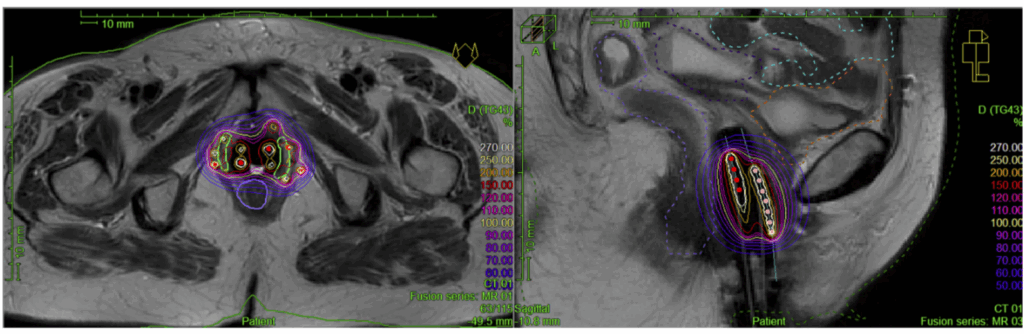
Imagine a 62-year-old woman, previously treated five years ago for stage IB, grade 2 endometrioid endometrial carcinoma. She had surgery followed by external beam radiation, but no brachytherapy. She’d been doing well—until now. A recent pelvic exam and MRI reveal a small, 1.5 cm lesion at the vaginal cuff. Biopsy confirms a localized recurrence.
Here’s where precision matters. She has no evidence of metastatic disease. Her functional status is good. The recurrence is small, accessible, and amenable to focused treatment. Her team decides to proceed with HDR brachytherapy. Over three outpatient sessions, she receives targeted radiation to the lesion site, with careful sparing of the rectum and bladder.
Fast-forward two years: she’s disease-free. Minimal side effects. No late toxicity. She goes back to her morning hikes and never misses a grandchild’s recital.
This isn’t fiction. It’s a very likely outcome seen in centers with experience, resources, and a patient-centric model. But it’s also a best-case scenario—clean recurrence, good anatomy, generous interval since first treatment. A lot of stars have to align.
But what about more complex cases?
Not all stories are that clean. Let’s flip the lens.
A 68-year-old woman with a high-grade serous carcinoma presents with a recurrence along the left pelvic sidewall—deep, infiltrative, and perilously close to the ureter and sigmoid colon. She had prior pelvic radiation and chemo two years ago. Surgery is not an option. Palliative chemo has limited impact.
Could SBRT help? In some cases, yes. With modern image guidance and adaptive planning, a high-dose, short-course treatment might offer pain relief and some local control. But the risks here are different: proximity to the bowel and ureter means there’s a real chance of complications—maybe not today, but months later. If her goal is palliation with minimal side effects, the balance might still lean toward SBRT. But if she’s hoping for a cure, her chances are markedly lower.
These are the judgment calls. This is where medicine becomes less about algorithms and more about values, priorities, and nuance.
What do the studies say?
The challenge with re-irradiation research is that it’s hard to standardize. You’re dealing with small, heterogenous populations, individualized treatment plans, and outcomes that depend as much on context as they do on technique. That said, the available data—while mostly retrospective—is encouraging in the right settings.
Take, for example, a 2018 study from the University of Toronto, which evaluated patients with recurrent gynecologic cancers treated with re-irradiation using brachytherapy. Local control rates were over 70% at two years, and serious toxicities were relatively uncommon in well-selected cases.
Or consider a multi-institutional review out of Europe analyzing IMRT-based re-irradiation for pelvic recurrences. It found that while toxicity was a legitimate concern, most patients tolerated the therapy well—and nearly half had meaningful symptom improvement or disease stabilization.
What we lack—at least for now—are randomized controlled trials specifically designed to compare re-irradiation to other options like systemic therapy or surgery. It’s unlikely we’ll ever get those large trials, frankly, because re-irradiation candidates are inherently diverse and not easy to randomize. But that doesn’t mean the data we do have is weak—it just means it’s contextual.
What’s happening in the research world?
Plenty. Several trials are underway to refine the science of re-irradiation in pelvic cancers. Some are investigating:
- How MRI-based adaptive planning can optimize brachytherapy dosing.
- Whether proton therapy can reduce late toxicity compared to IMRT.
- The use of radiosensitizers or immunotherapy in conjunction with re-irradiation to improve efficacy without increasing harm.
There’s also growing interest in biological dose modeling—using genomic data to predict how a tumor (and a patient’s normal tissues) might respond to re-irradiation. This is the cutting edge: personalizing not just whether to re-irradiate, but how, where, and with what kind of supporting agents.
So what should patients make of all this?
Here’s the takeaway: you’re not walking into an experimental wilderness. Re-irradiation has precedent. It has protocols. It has outcomes worth considering—and worth striving for. But it’s not a magic bullet. It demands precise planning, honest discussion, and careful follow-up.
It also demands a team that knows how to navigate this terrain—because this is not generalist oncology. It’s subspecialty care that benefits immensely from being delivered at high-volume centers where radiation oncology, medical oncology, surgery, and palliative care work as a unit.
Guidelines and Recommendations
Let’s say you’re a clinician staring down a recurrence in a previously irradiated pelvis. Or maybe you’re a patient, newly told that your endometrial cancer is back—and this time, the treatment that once worked is now part of the complication. The natural question that follows is: So, what do the guidelines say? Is there a standard approach for re-irradiation?
You’d think, with something this serious, there’d be a detailed rulebook. A flowchart. Something official. But here’s the truth: while there are recommendations and consensus statements, formal, universally accepted guidelines for re-irradiation in endometrial cancer remain surprisingly sparse. Not because the field isn’t evolving—but because it’s evolving faster than the institutions can codify.
Still, that doesn’t mean you’re navigating in the dark. Several professional organizations have weighed in on the broader principles, and there are institutional protocols that provide a reliable scaffolding for making decisions.
What do the big societies say?
Organizations like ASTRO (American Society for Radiation Oncology), ESGO (European Society of Gynaecological Oncology), and ESMO (European Society for Medical Oncology) all acknowledge re-irradiation as a possible strategy in recurrent endometrial cancer, particularly for patients with isolated pelvic or vaginal recurrences. But they stop short of issuing hard-and-fast rules.
Why? Because the decision to re-irradiate is too individualized. It depends on the specifics of prior radiation (what dose, what field, what technique), the exact location and size of recurrence, patient anatomy, comorbidities, goals of care—the list goes on. There is no “one-size-fits-all” model that works across the full spectrum of recurrence scenarios.
Instead, the guidance tends to focus on principles:
- Re-irradiation should only be considered in centers with high expertise and access to advanced planning technologies.
- It should involve multidisciplinary review, including surgical, medical, and radiation oncology.
- There must be documented evidence of disease localization, typically with PET/CT or MRI, to confirm that the recurrence is suitable for localized therapy.
- The patient must be fully counseled on risks, benefits, and alternatives, with a clear understanding that this isn’t “standard” radiation therapy—it’s a second strike on already irradiated tissue.
So in practice, guidelines serve more as frameworks for thought than directives. They encourage rigor, caution, and personalization—not rote application of algorithms.
Are there any consensus documents or position papers?
Yes, though they’re often tucked away in literature more broadly addressing recurrent gynecologic cancers. One standout is the ESGO-ESTRO-ESP consensus on management of endometrial carcinoma, which explicitly recognizes re-irradiation as an option in select cases, particularly where other modalities (like surgery or systemic therapy) are contraindicated or have failed.
These documents emphasize:
- The value of brachytherapy for central recurrences, especially in patients who did not receive it previously.
- The potential role of advanced EBRT techniques for lateral or nodal recurrences, provided cumulative organ doses can be safely managed.
- The absolute necessity of patient selection—not just biologically and anatomically, but psychologically and socially. Is the patient willing and able to commit to close follow-up? Do they have the support system needed to manage potential complications?
These are the kinds of questions that guidelines won’t answer directly—but that clinicians must, if they’re going to do right by their patients.
What about institutional protocols?
Large academic centers and high-volume cancer hospitals often have their own protocols for re-irradiation—not because they’re trying to replace national guidelines, but because they’ve developed internal standards based on experience.
These protocols typically include:
- Pre-treatment review of all prior radiation records, including dose-volume histograms.
- Cumulative dose modeling to critical structures, often with specialized software.
- A requirement for multidisciplinary tumor board approval before proceeding.
- Patient education sessions that outline not only potential benefits but also the full spectrum of acute and late toxicities, and what interventions may be necessary if complications arise.
This institutional rigor is crucial. Re-irradiation is one of those decisions that can’t be walked back. It has to be made with clear eyes, full data, and shared ownership between the patient and care team.
Where are the gray zones?
They’re everywhere. For example: What if a patient had EBRT ten years ago but now presents with a new central recurrence? Can we treat her like a new patient? Not exactly—some radiation effects persist indefinitely—but with a decade of interval and modern techniques, many centers would consider her a candidate for HDR brachytherapy, possibly curative.
What if her recurrence is near the rectum, which already absorbed its maximum tolerable dose? Can we re-treat with SBRT? Maybe—but only if the dose sculpting can thread the needle with enough precision to spare the rectal wall. These aren’t academic puzzles—they’re daily dilemmas. And in many cases, the guidelines simply say: Consider, discuss, and individualize.
So how do patients and clinicians use these recommendations?
As conversation starters. As boundary lines, not blueprints. They set the stage for honest, data-informed discussions between doctors and patients. No one should go into re-irradiation thinking there’s a guaranteed outcome—but they also shouldn’t assume that the lack of a rigid guideline means there’s no hope.
The truth is, guidelines are there to protect patients—but they’re also a signpost of how complex and rapidly evolving the field really is. Re-irradiation used to be a clinical cul-de-sac. Now, it’s a detour—a hard, careful path that may still lead somewhere worth going.
And for those wondering where to draw the line on further treatment, Terminal Cancer Couloir offers a candid, compassionate look at hard decisions.
Future Directions and Innovations
If you’ve followed along this far, you’ve seen that re-irradiation for endometrial cancer sits at a fascinating crossroads of opportunity and challenge. It’s an area where past limitations have given way to new technology, and where old fears—of toxicity, irreversibility, futility—are now met with cautious optimism. But the story doesn’t end here. In fact, the most exciting chapter may still be unwritten.
So what does the future hold? How might ongoing scientific and technological advances reshape how we think about—and deliver—re-irradiation in endometrial cancer? And perhaps most importantly, what new hope can these developments offer patients facing recurrence?
The Promise of Technological Precision
Radiation oncology has come a long way from those early days of crude fields and generous margins. But even now, the quest for greater precision continues. The future of re-irradiation lies in pushing the boundaries of imaging and delivery so that we can escalate tumor dose while continuing to spare normal tissues—especially those previously radiated.
One of the most promising innovations is MRI-guided adaptive radiotherapy. Unlike traditional CT-based planning, MRI offers unparalleled soft tissue contrast, allowing real-time visualization of tumors and organs at risk. This means radiation beams can be adjusted daily—or even during a session—to account for shifts in anatomy, organ filling, or tumor response. For re-irradiation, where millimeters matter, this could be a game-changer.
Another leap forward is biological dose painting—a technique that uses functional imaging (like PET scans) to identify the most active or resistant parts of a tumor and deliver higher doses specifically there. Instead of a uniform dose across the entire recurrence, this approach personalizes the radiation dose distribution within the tumor itself, potentially overcoming radioresistance and improving control without increasing toxicity.
Harnessing the Power of Particle Therapy
We touched earlier on proton and carbon ion therapy, but the field continues to evolve rapidly. These particle therapies offer physical and biological advantages that could redefine re-irradiation safety and efficacy.
Protons, with their Bragg peak phenomenon, can sharply reduce the dose beyond the tumor, limiting collateral damage. Carbon ions not only do this but also have a higher relative biological effectiveness (RBE), meaning they might be more effective at killing radioresistant tumor cells.
Clinical trials are ongoing, and while these therapies remain resource-intensive and expensive, their role in treating recurrent endometrial cancer is being actively explored. We may soon see particle therapy becoming more accessible, and more integrated into re-irradiation protocols, particularly for challenging anatomical sites or aggressive histologies.
Personalizing Treatment Through Molecular Insights
What if we could predict, before treatment, which tumors are likely to respond to re-irradiation? Or which patients are at greatest risk for severe toxicity? The field of radiogenomics and molecular profiling is advancing toward these goals.
By analyzing genetic markers, tumor mutations, and the tumor microenvironment, researchers hope to tailor radiation doses and schedules uniquely to each patient’s biology. For example, tumors with mutations that confer radioresistance might benefit from dose escalation or combination with radiosensitizing agents. Conversely, patients with genetic susceptibilities to radiation damage might be spared aggressive re-irradiation or receive enhanced protective strategies.
This personalized approach has the potential to dramatically improve outcomes while minimizing harm. It’s precision medicine brought to the realm of re-irradiation.
Combining Re-irradiation with Systemic Therapies
Radiation no longer works in isolation. The burgeoning field of immunotherapy—harnessing the body’s own immune system to fight cancer—offers exciting possibilities when combined with re-irradiation.
Radiation can increase tumor antigen presentation and modulate the tumor microenvironment, potentially making tumors more susceptible to immune checkpoint inhibitors and other immunomodulators. Clinical trials are underway investigating these combinations in recurrent gynecologic cancers.
Moreover, targeted therapies that sensitize tumors to radiation or protect normal tissues are also being explored, offering a way to push the therapeutic ratio further in favor of the patient.
Artificial Intelligence and Treatment Planning
AI isn’t just hype—it’s already reshaping radiation oncology. Algorithms can help contour tumors and organs more quickly and accurately, predict patient-specific toxicity risks, and even recommend optimal dose distributions based on thousands of prior cases.
For re-irradiation, where planning is complex and errors costly, AI-assisted decision support could become indispensable. Imagine a system that flags unsafe cumulative doses, suggests alternative beam angles, or forecasts late toxicity risks with unprecedented precision.
What does this mean for patients?
In a word: hope. Not unfounded optimism, but evidence-based progress. The future holds a promise that re-irradiation can become safer, more effective, and more personalized than ever before.
But let’s be clear: these advances will not replace the fundamental need for expert clinical judgment, multidisciplinary collaboration, and patient-centered decision-making. The human element—empathy, communication, shared goals—remains paramount.
As we look forward, we can be cautiously optimistic that the difficult question, “Can we safely give radiation again?”, will increasingly be met with answers grounded in cutting-edge science, evolving technology, and an unwavering commitment to improving patient lives.
Frequently Asked Questions (FAQs)
1. What is re-irradiation, and why is it used in endometrial cancer?
Re-irradiation involves delivering radiation to an area that was previously treated—typically due to a local recurrence of endometrial cancer. It’s considered when surgery or systemic therapy isn’t an option, and can be used with curative intent or for symptom relief, depending on the situation.
2. How is re-irradiation different from the first round of radiation?
Initial radiation treats tissues that haven’t been exposed before, allowing for a wider safety margin. Re-irradiation targets tissue already stressed by earlier treatment, so the approach requires greater precision and comes with a narrower therapeutic window—and higher risks.
3. What are the main risks and side effects of re-irradiation?
Because tissues are already sensitized, there’s an increased risk of both short-term effects like fatigue and bowel or bladder irritation, and long-term complications such as fibrosis, fistulas, organ damage, or nerve injury. That’s why patient selection and careful planning are critical.
4. Who qualifies for re-irradiation treatment?
Eligibility depends on several factors: where and how large the recurrence is, how much time has passed since initial treatment, prior radiation dose, current health status, and whether the disease is localized. A multidisciplinary team typically reviews each case in detail.
5. Does re-irradiation actually work—and how effective is it?
When used appropriately, re-irradiation can control localized recurrences in 60–80% of cases over 2–5 years. Success depends on tumor biology, recurrence size, and prior treatment history. It may also improve symptoms and quality of life, even when cure isn’t possible.
6. What options exist to improve safety and outcomes with re-irradiation?
Advanced techniques like IMRT, SBRT, brachytherapy, and MRI-guided radiotherapy allow for better targeting and reduced harm to healthy tissues. In some cases, re-irradiation is paired with chemotherapy or immunotherapy to boost effectiveness. Follow-up care includes frequent monitoring to catch late effects early and manage long-term well-being.
Closing Thoughts
Re-irradiation for endometrial cancer represents a complex, evolving frontier—one that combines cutting-edge technology with nuanced clinical judgment and deeply personal decision-making. It is not without risks, but for select patients, it offers a meaningful chance at disease control, symptom relief, and even cure.
Navigating this terrain demands collaboration between experienced specialists and patients who are informed, supported, and empowered. The decision to re-irradiate is never simple, but with advancing tools, better biological understanding, and a patient-centered approach, it can be made with greater confidence and hope.
Remember, every patient’s story is unique. What matters most is finding the path that aligns best with their values, goals, and circumstances. And as science progresses, so too does our ability to offer tailored, effective care in the face of recurrence.
If you or a loved one are facing this decision, take heart in the progress made—and take time to ask questions, seek expertise, and surround yourself with a team that listens as much as it treats.
The journey is challenging, but you are not alone.